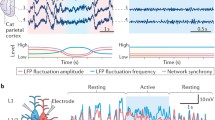
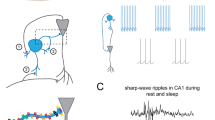
Abstract
Cognitive and behavioral acts go along with highly coordinated spatiotemporal activity patterns in neuronal networks. Most of these patterns are synchronized by coherent membrane potential oscillations within and between local networks. By entraining multiple neurons into a common time regime, such network oscillations form a critical interface between cellular activity and large-scale systemic functions. Synaptic integrity is altered in neurodegenerative diseases, and it is likely that this goes along with characteristic changes of coordinated network activity. This notion is supported by EEG recordings from human patients and from different animal models of such disorders. However, our knowledge about the pathophysiology of network oscillations in neurodegenerative diseases is surprisingly incomplete, and increased research efforts are urgently needed. One complicating factor is the pronounced diversity of network oscillations between different brain regions and functional states. Pathological changes must, therefore, be analyzed separately in each condition and affected area. However, cumulative evidence from different diseases may result, in the future, in more unifying “oscillopathy” concepts of neurodegenerative diseases. In this review, we report present evidence for pathological changes of network oscillations in Alzheimer’s disease (AD), one of the most prominent and challenging neurodegenerative disorders. The heterogeneous findings from AD are contrasted to Parkinson’s disease, where motor-related changes in specific frequency bands do already fulfill criteria of a valid biomarker.

Similar content being viewed by others
References
Abramov, E., Dolev, I., Fogel, H., Ciccotosto, G. D., Ruff, E., & Slutsky, I. (2009). Amyloid-beta as a positive endogenous regulator of release probability at hippocampal synapses. Nature Neuroscience, 12, 1567–1576.
Adaya-Villanueva, A., Ordaz, B., Balleza-Tapia, H., Márquez-Ramos, A., & Peña-Ortega, F. (2010). Beta-like hippocampal network activity is differentially affected by amyloid beta peptides. Peptides, 31, 1761–1766.
Amatniek, J. C., Hauser, W. A., DelCastillo-Castaneda, C., Jacobs, D. M., Marder, K., Bell, K., et al. (2006). Incidence and predictors of seizures in patients with Alzheimer’s disease. Epilepsia, 47, 867–872.
Babiloni, C., Binetti, G., Cassetta, E., Cerboneschi, D., Dal Forno, G., Del Percio, C., et al. (2004). Mapping distributed sources of cortical rhythms in mild Alzheimer’s disease. A multicentric EEG study. Neuroimage, 22, 57–67.
Bähner, F., Weiss, E. K., Birke, G., Maier, N., Schmitz, D., Rudolph, U., et al. (2011). Cellular correlate of assembly formation in oscillating hippocampal networks in vitro. Proceedings of the National Academy of Sciences of the United States of America, 108, E607–E616.
Bakker, A., Krauss, G. L., Albert, M. S., Speck, C. L., Jones, L. R., Stark, C. E., et al. (2012). Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron, 74, 467–474.
Bartus, R. T., Dean, R. L, 3rd, Beer, B., & Lippa, A. S. (1982). The cholinergic hypothesis of geriatric memory dysfunction. Science, 217, 408–414.
Berger, H. (1929). Über das Elektroenkephalogramm des Menschen. Archiv für Psychiatrie und Nervenkrankheiten, 87, 527–570.
Besthorn, C., Zerfass, R., Geiger-Kabisch, C., Sattel, H., Daniel, S., Schreiter-Gasser, U., & Förstl, H. (1997). Discrimination of Alzheimer’s disease and normal aging by EEG data. Electroencephalography and Clinical Neurophysiology, 103, 241–248.
Blatow, M., Rozov, A., Katona, I., Hormuzdi, S. G., Meyer, A. H., Whittington, M. A., et al. (2003). A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron, 38, 805–817.
Bokde, A. L., Ewers, M., & Hampel, H. (2009). Assessing neuronal networks: understanding Alzheimer’s disease. Progress in Neurobiology, 89, 125–133.
Bosboom, J. L., Stoffers, D., Stam, C. J., Berendse, H. W., & Wolters, E Ch. (2009). Cholinergic modulation of MEG resting-state oscillatory activity in Parkinson’s disease related dementia. Clinical Neurophysiology, 120, 910–915.
Bosboom, J. L., Stoffers, D., Stam, C. J., van Dijk, B. W., Verbunt, J., Berendse, H. W., & Wolters, E Ch. (2006). Resting state oscillatory brain dynamics in Parkinson’s disease: An MEG study. Clinical Neurophysiology, 117, 2521–2531.
Böttger, D., Herrmann, C. S., & von Cramon, D. Y. (2002). Amplitude differences of evoked alpha and gamma oscillations in two different age groups. International Journal of Psychophysiology, 45, 245–251.
Brown, P., & Williams, D. (2005). Basal ganglia local field potential activity: Character and functional significance in the human. Clinical Neurophysiology, 116, 2510–2519.
Busche, M. A., Chen, X., Henning, H. A., Reichwald, J., Staufenbiel, M., Sakmann, B., & Konnerth, A. (2012). Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America, 109, 8740–8745.
Busche, M. A., Eichhoff, G., Adelsberger, H., Abramowski, D., Wiederhold, K. H., Haass, C., et al. (2008). Clusters of hyperactive neurons near amyloid plaques in a mouse model of Alzheimer’s disease. Science, 321, 1686–1689.
Buzsáki, G. (2006). Rhythms of the brain. Oxford, New York: Oxford University Press.
Buzsáki, G. (2002). Theta oscillations in the hippocampus. Neuron, 33, 325–340.
Buzsáki, G., & Draguhn, A. (2004). Neuronal oscillations in cortical networks. Science, 304, 1926–1929.
Buzsáki, G., & Gage, F. H. (1989). The cholinergic nucleus basalis: A key structure in neocortical arousal. EXS, 57, 159–171.
Buzsáki, G., Horvath, Z., Urioste, R., Hetke, J., & Wise, K. (1992). High-frequency network oscillation in the hippocampus. Science, 256, 1025–1027.
Bylsma, F. W., Peyser, C. E., Folstein, S. E., Folstein, M. F., Ross, C., & Brandt, J. (1994). EEG power spectra in Huntington’s disease: Clinical and neuropsychological correlates. Neuropsychologia, 32, 137–150.
Cardin, J. A., Carlen, M., Meletis, K., Knoblich, U., Zhang, F., Deisseroth, K., et al. (2009). Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature, 459, 663–667.
Caso, F., Cursi, M., Magnani, G., Fanelli, G., Falautano, M., Comim, G., et al. (2012). Quantitative EEG and LORETA: Valuable tools in discerning FTD from AD? Neurobiology of Aging, 33, 2343–2356.
Cea-del Rio, C. A., Lawrence, J. J., Erdelyi, F., Szabo, G., & McBain, C. J. (2011). Cholinergic modulation amplifies the intrinsic oscillatory properties of CA1 hippocampal cholecystokinin-positive interneurons. Journal of Physiology, 589, 609–627.
Celone, K. A., Calhoun, V. D., Dickerson, B. C., Atri, A., Chua, E. F., Miller, S. L., et al. (2006). Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: An independent component analysis. Journal of Neuroscience, 26, 10222–10231.
Chen, C. C., Lin, W. Y., Chan, H. L., Hsu, Y. T., Tu, P. H., Lee, S. T., et al. (2011). Stimulation of the subthalamic region at 20 Hz slows the development of grip force in Parkinson’s disease. Experimental Neurology, 231, 91–96.
Chen, C. C., Litvak, V., Gilbertson, T., Kühn, A., Lu, C. S., Lee, S. T., et al. (2007). Excessive synchronization of basal ganglia neurons at 20 Hz slows movement in Parkinson’s disease. Experimental Neurology, 205, 214–221.
Chiaramonti, R., Muscas, G. C., Paganini, M., Müller, T. J., Fallgatter, A. J., Versari, A., & Strik, W. K. (1997). Correlations of topographical EEG features with clinical severity in mild and moderate dementia of Alzheimer type. Neuropsychobiology, 36, 153–158.
Claus, J. J., Strijers, R. L., Jonkman, E. J., Ongerboer de Visser, B. W., Jonker, C., Walstra, G. J., et al. (1999). The diagnostic value of electroencephalography in mild senile Alzheimer’s disease. Clinical Neurophysiology, 110, 825–832.
Cloud, L. J., Rosenblatt, A., Margolis, R. L., Ross, C. A., Pillai, J. A., Corey-Bloom, J., et al. (2012). Seizures in juvenile Huntington’s disease: Frequency and characterization in a multicenter cohort. Movement Disorders, 27, 1797–1800.
Cook, I. A., & Leuchter, A. F. (1996). Synaptic dysfunction in Alzheimer’s disease: Clinical assessment using quantitative EEG. Behavioural Brain Research, 78, 15–23.
Cross, A. J. (1990). Serotonin in Alzheimer-type dementia and other dementing illnesses. Annals of the New York Academy of Sciences, 600, 405–415.
Cummins, T. D., Broughton, M., & Finnigan, S. (2008). Theta oscillations are affected by amnestic mild cognitive impairment and cognitive load. International Journal of Psychophysiology, 70, 75–81.
Curley, A. A., & Lewis, D. A. (2012). Cortical basket cell dysfunction in schizophrenia. Journal of Physiology, 590, 715–724.
De Felice, F. G., Velasco, P. T., Lambert, M. P., Viola, K., Fernandez, S. J., Ferreira, S. T., & Klein, W. L. (2007). Abeta oligomers induce neuronal oxidative stress through an NMDA receptor-dependent mechanism that is blocked by the Alzheimer’s drug memantine. Journal of Biological Chemistry, 282, 11590–11601.
de Tommaso, M., De Carlo, F., Difruscolo, O., Massafra, R., Sciruicchio, V., & Bellotti, R. (2003). Detection of subclinical brain electrical activity changes in Huntington’s disease using artificial neural networks. Clinical Neurophysiology, 114, 1237–1245.
Détári, L., Rasmusson, D. D., & Semba, K. (1999). The role of basal forebrain neurons in tonic and phasic activation of the cerebral cortex. Progress in Neurobiology, 58, 249–277.
Di Lazzaro, V., Oliviero, A., Pilato, F., Saturno, E., Dileone, M., Marra, C., et al. (2004). Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer’s disease. Journal of Neurology, Neurosurgery and Psychiatry, 75, 555–559.
Dougherty, J. J., Wu, J., & Nichols, R. A. (2003). Beta-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex. Journal of Neuroscience, 23, 6740–6747.
Draguhn, A., Traub, R. D., Schmitz, D., & Jefferys, J. G. (1998). Electrical coupling underlies high-frequency oscillations in the hippocampus in vitro. Nature, 394, 189–192.
Dringenberg, H. C. (2000). Alzheimer’s disease: More than a ‘cholinergic disorder’—Evidence that cholinergic–monoaminergic interactions contribute to EEG slowing and dementia. Behavioural Brain Research, 115, 235–249.
Driver, J. E., Racca, C., Cunningham, M. O., Towers, S. K., Davies, C. H., Whittington, M. A., & LeBeau, F. E. (2007). Impairment of hippocampal gamma-frequency oscillations in vitro in mice overexpressing human amyloid precursor protein (APP). European Journal of Neuroscience, 26, 1280–1288.
Düzel, E., Penny, W. D., & Burgess, N. (2010). Brain oscillations and memory. Current Opinion in Neurobiology, 20, 143–149.
Ego-Stengel, V., & Wilson, M. A. (2010). Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus, 20, 1–10.
Engel, A. K., Fries, P., & Singer, W. (2001). Dynamic predictions: Oscillations and synchrony in top-down processing. Nature Reviews Neuroscience, 2, 704–716.
Eusebio, A., Chen, C. C., Lu, C. S., Lee, S. T., Tsai, C. H., Limousin, P., et al. (2008). Effects of low-frequency stimulation of the subthalamic nucleus on movement in Parkinson’s disease. Experimental Neurology, 209, 125–130.
Fell, J., & Axmacher, N. (2011). The role of phase synchronization in memory processes. Nature Reviews Neuroscience, 12, 105–118.
Fell, J., Klaver, P., Elger, C. E., & Fernández, G. (2002). The interaction of rhinal cortex and hippocampus in human declarative memory formation. Reviews in the Neurosciences, 13, 299–312.
Fell, J., Staresina, B. P., Do Lam, A. T., Widman, G., Helmstaedter, C., Elger, C. E., & Axmacher, N. (2013). Memory modulation by weak synchronous deep brain stimulation: A pilot study. Brain Stimul, 6, 270–273.
Filippini, N., MacIntosh, B. J., Hough, M. G., Goodwin, G. M., Frisoni, G. B., Smith, S. M., et al. (2009). Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proceedings of the National Academy of Sciences of the United States of America, 106, 7209–7214.
Foffani, G., Priori, A., Egidi, M., Rampini, P., Tamma, F., Caputo, E., et al. (2003). 300-Hz subthalamic oscillations in Parkinson’s disease. Brain, 126, 2153–2163.
Frank, M. J. (2006). Hold your horses: A dynamic computational role for the subthalamic nucleus in decision making. Neural Networks, 19, 1120–1136.
Freund, T. F., & Buzsáki, G. (1996). Interneurons of the hippocampus. Hippocampus, 6, 347–470.
Fries, P. (2005). A mechanism for cognitive dynamics: Neuronal communication through neuronal coherence. Trends in Cognitive Sciences, 9, 474–480.
García-Cabrero, A. M., Guerrero-López, R., Giráldez, B. G., Llorens-Martín, M., Avila, J., Serratosa, J. M., & Sánchez, M. P. (2013). Hyperexcitability and epileptic seizures in a model of frontotemporal dementia. Neurobiology of Diseases, 58, 200–208.
Girardeau, G., Benchenane, K., Wiener, S. I., Buzsáki, G., & Zugaro, M. B. (2009). Selective suppression of hippocampal ripples impairs spatial memory. Nature Neuroscience, 12, 1222–1223.
Goutagny, R., & Krantic, S. (2013). Hippocampal oscillatory activity in Alzheimer’s disease: Toward the identification of early biomarkers? Aging and Disease, 4, 134–140.
Grady, C. L., McIntosh, A. R., Beig, S., Keightley, M. L., Burian, H., & Black, S. E. (2003). Evidence from functional neuroimaging of a compensatory prefrontal network in Alzheimer’s disease. Journal of Neuroscience, 23, 986–993.
Gregory, R. (2008). Pedunculopontine nucleus stimulation for people with Parkinson’s disease? A clinical perspective. British Journal of Neurosurgery, 22(Suppl 1), S13–S15.
Grillner, S. (2006). Biological pattern generation: The cellular and computational logic of networks in motion. Neuron, 52, 751–766.
Gruber, T., Müller, M. M., & Keil, A. (2002). Modulation of induced gamma band responses in a perceptual learning task in the human EEG. Journal of Cognitive Neuroscience, 14, 732–744.
Gruber, T., Müller, M. M., Keil, A., & Elbert, T. (1999). Selective visual–spatial attention alters induced gamma band responses in the human EEG. Clinical Neurophysiology, 110, 2074–2085.
Haier, R. J., Alkire, M. T., White, N. S., Uncapher, M. R., Head, E., Lott, I. T., & Cotman, C. W. (2003). Temporal cortex hypermetabolism in Down syndrome prior to the onset of dementia. Neurology, 61, 1673–1679.
Hammond, C., Bergman, H., & Brown, P. (2007). Pathological synchronization in Parkinson’s disease: Networks, models and treatments. Trends in Neurosciences, 30, 357–364.
Hardy, J. A., & Higgins, G. A. (1992). Alzheimer’s disease: The amyloid cascade hypothesis. Science, 256, 184–185.
Hatashita, S., & Yamasaki, H. (2013). Diagnosed mild cognitive impairment due to Alzheimer’s disease with PET biomarkers of beta amyloid and neuronal dysfunction. PLoS ONE, 8, e66877.
Hermann, D., Both, M., Ebert, U., Gross, G., Schoemaker, H., Draguhn, A., et al. (2009). Synaptic transmission is impaired prior to plaque formation in amyloid precursor protein-overexpressing mice without altering behaviorally-correlated sharp wave–ripple complexes. Neuroscience, 162, 1081–1090.
Hermann, D., Mezler, M., Müller, M. K., Wicke, K., Gross, G., Draguhn, A., et al. (2013). Synthetic Aβ oligomers (Aβ(1-42) globulomer) modulate presynaptic calcium currents: Prevention of Aβ-induced synaptic deficits by calcium channel blockers. European Journal of Pharmacology, 702, 44–55.
Herrmann, C. S., & Demiralp, T. (2005). Human EEG gamma oscillations in neuropsychiatric disorders. Clinical Neurophysiology, 116, 2719–2733.
Igarashi, K. M., Lu, L., Colgin, L. L., Moser, M. B., & Moser, E. I. (2014). Coordination of entorhinal–hippocampal ensemble activity during associative learning. Nature, 510, 143–147.
Jelic, V., Blomberg, M., Dierks, T., Basun, H., Shigeta, M., Julin, P., et al. (1998). EEG slowing and cerebrospinal fluid tau levels in patients with cognitive decline. Neuroreport, 9, 157–160.
Jelic, V., Julin, P., Shigeta, M., Nordberg, A., Lannfelt, L., Winblad, B., & Wahlund, L. O. (1997). Apolipoprotein E epsilon4 allele decreases functional connectivity in Alzheimer’s disease as measured by EEG coherence. Journal of Neurology, Neurosurgery and Psychiatry, 63, 59–65.
Jensen, O., Kaiser, J., & Lachaux, J. P. (2007). Human gamma-frequency oscillations associated with attention and memory. Trends in Neurosciences, 30, 317–324.
Jeong, J. (2004). EEG dynamics in patients with Alzheimer’s disease. Clinical Neurophysiology, 115, 1490–1505.
Jyoti, A., Plano, A., Riedel, G., & Platt, B. (2010). EEG, activity, and sleep architecture in a transgenic AβPPswe/PSEN1A246E Alzheimer’s disease mouse. Journal of Alzheimer’s Disease, 22, 873–887.
Kaiser, J., Ripper, B., Birbaumer, N., & Lutzenberger, W. (2003). Dynamics of gamma-band activity in human magnetoencephalogram during auditory pattern working memory. Neuroimage, 20, 816–827.
Kamenetz, F., Tomita, T., Hsieh, H., Seabrook, G., Borchelt, D., Iwatsubo, T., et al. (2003). APP processing and synaptic function. Neuron, 37, 925–937.
Kawaguchi, Y., & Kondo, S. (2002). Parvalbumin, somatostatin and cholecystokinin as chemical markers for specific GABAergic interneuron types in the rat frontal cortex. Journal of Neurocytology, 31, 277–287.
Kelly, B. L., & Ferreira, A. (2006). Beta-Amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. Journal of Biological Chemistry, 281, 28079–28089.
Kelly, B. L., & Ferreira, A. (2007). Beta-amyloid disrupted synaptic vesicle endocytosis in cultured hippocampal neurons. Neuroscience, 147, 60–70.
Klausberger, T. (2009). GABAergic interneurons targeting dendrites of pyramidal cells in the CA1 area of the hippocampus. European Journal of Neuroscience, 30, 947–957.
Klausberger, T., & Somogyi, P. (2008). Neuronal diversity and temporal dynamics: The unity of hippocampal circuit operations. Science, 321, 53–57.
Kotzauer, N., & Katz, R. (2013). Regulatory innovation and drug development for early-stage Alzheimer‘s disease. New England Journal of Medicine, 368, 1169–1171.
Kühn, A. A., Trottenberg, T., Kivi, A., Kupsch, A., Schneider, G. H., & Brown, P. (2005). The relationship between local field potential and neuronal discharge in the subthalamic nucleus of patients with Parkinson’s disease. Experimental Neurology, 194, 212–220.
Kumar-Singh, S., Dewachter, I., Moechars, D., Lübke, U., De Jonghe, C., Ceuterick, C., et al. (2000). Behavioral disturbances without amyloid deposits in mice overexpressing human amyloid precursor protein with Flemish (A692G) or Dutch (E693Q) mutation. Neurobiology of Diseases, 7, 9–22.
Lacor, P. N., Bruniel, M. C., Furlow, P. W., Sanz Clemente, A., Velasco, P. T., Wood, M., et al. (2007). Aß oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. Journal of Neuroscience, 27, 796–807.
LaFerla, F. M. (2010). Pathways linking Abeta and tau pathologies. Biochemical Society Transactions, 38, 993–995.
LaFerla, F. M., Tinkle, B. T., Bieberich, C. J., Haudenschild, C. C., & Jay, G. (1995). The Alzheimer’s A beta peptide induces neurodegeneration and apoptotic cell death in transgenic mice. Nature Genetics, 9, 21–30.
Lalonde, R., Dumont, M., Staufenbiel, M., & Strazielle, C. (2005). Neurobehavioral characterization of APP23 transgenic mice with the SHIRPA primary screen. Behavioural Brain Research, 157, 91–98.
Larner, A. J. (2010). Epileptic seizures in AD patients. Neuromolecular Medicine, 12, 71–77.
Lasztóczi, B., & Klausberger, T. (2014). Layer-specific GABAergic control of distinct gamma oscillations in the CA1 hippocampus. Neuron, 81, 1126–1139.
Lee, H., Fell, J., & Axmacher, N. (2013). Electrical engram: How deep brain stimulation affects memory. Trends in Cognitive Sciences, 17, 574–584.
Lewis, D. A., Curley, A. A., Glausier, J. R., & Volk, D. W. (2012). Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in Neurosciences, 35, 57–67.
Lindau, M., Jelic, V., Johansson, S. E., Andersen, C., Wahlund, L. O., & Almkvist, O. (2003). Quantitative EEG abnormalities and cognitive dysfunctions in frontotemporal dementia and Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders, 15, 106–114.
Locatelli, T., Cursi, M., Liberati, D., Franceschi, M., & Comi, G. (1998). EEG coherence in Alzheimer’s disease. Electroencephalography and Clinical Neurophysiology, 106, 229–237.
López-Azcárate, J., Tainta, M., Rodríguez-Oroz, M. C., Valencia, M., González, R., Guridi, J., et al. (2010). Coupling between beta and high-frequency activity in the human subthalamic nucleus may be a pathophysiological mechanism in Parkinson’s disease. Journal of Neuroscience, 30, 6667–6677.
Lozano, A. M., & Lipsman, N. (2013). Probing and regulating dysfunctional circuits using deep brain stimulation. Neuron, 77, 406–424.
Lustig, C., Snyder, A. Z., Bhakta, M., O’Brien, K. C., McAvoy, M., Raichle, M. E., et al. (2003). Functional deactivations: Change with age and dementia of the Alzheimer type. Proceedings of the National Academy of Sciences of the United States of America, 100, 14504–14509.
Mann, E. O., & Paulsen, O. (2007). Role of GABAergic inhibition in hippocampal network oscillations. Trends in Neurosciences, 30, 343–349.
Mendez, M., & Lim, G. (2003). Seizures in elderly patients with dementia: Epidemiology and management. Drugs and Aging, 20, 791–803.
Metherate, R., Cox, C. L., & Ashe, J. H. (1992). Cellular bases of neocortical activation: Modulation of neural oscillations by the nucleus basalis and endogenous acetylcholine. Journal of Neuroscience, 12, 4701–4711.
Mezler, M., Barghorn, S., Schoemaker, H., Gross, G., & Nimmrich, V. (2012). A β-amyloid oligomer directly modulates P/Q-type calcium currents in Xenopus oocytes. British Journal of Pharmacology, 165, 1572–1583.
Miller, S. L., Celone, K., DePeau, K., Diamond, E., Dickerson, B. C., Rentz, D., et al. (2008). Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proceedings of the National Academy of Sciences of the United States of America, 105, 2181–2186.
Minkeviciene, R., Rheims, S., Dobszay, M. B., Zilberter, M., Hartikainen, J., Fülöp, L., et al. (2009). Amyloid beta-induced neuronal hyperexcitability triggers progressive epilepsy. Journal of Neuroscience, 29, 3453–3462.
Moechars, D., Lorent, K., & Van Leuven, F. (1999). Premature death in transgenic mice that overexpress a mutant amyloid precursor protein is preceded by severe neurodegeneration and apoptosis. Neuroscience, 91, 819–830.
Mondadori, C. R., Buchmann, A., Mustovic, H., Schmidt, C. F., Boesiger, P., Nitsch, R. M., et al. (2006). Enhanced brain activity may precede the diagnosis of Alzheimer’s disease by 30 years. Brain, 129, 2908–2922.
Montez, T., Poil, S. S., Jones, B. F., Manshanden, I., Verbunt, J. P., van Dijk, B. W., et al. (2009). Altered temporal correlations in parietal alpha and prefrontal theta oscillations in early-stage Alzheimer disease. Proceedings of the National Academy of Sciences of the United States of America, 106, 1614–1619.
Montplaisir, J., Petit, D., Gauthier, S., Gaudreau, H., & Décary, A. (1998). Sleep disturbances and eeg slowing in Alzheimer’s disease. Sleep Research Online, 1, 147–151.
Moraes Wdos, S., Poyares, D. R., Guilleminault, C., Ramos, L. R., Bertolucci, P. H., & Tufik, S. (2006). The effect of donepezil on sleep and REM sleep EEG in patients with Alzheimer disease: A double-blind placebo-controlled study. Sleep, 29, 199–205.
Neary, D., Snowden, J. S., Gustafson, L., Passant, U., Stuss, D., Black, S., et al. (1998). Frontotemporal lobar degeneration. A consensus on clinical diagnostic criteria. Neurology, 51, 1546–1554.
Nimmrich, V., & Ebert, U. (2009). Is Alzheimer’s disease a result of presynaptic failure? Synaptic dysfunctions induced by oligomeric beta-amyloid. Reviews in the Neurosciences, 20, 1–12.
Nimmrich, V., Grimm, C., Draguhn, A., Barghorn, S., Lehmann, A., Schoemaker, H., et al. (2008). Amyloid ß oligomers (Aß 1-42 globulomer) suppress spontaneous synaptic activity by inhibition of P/Q calcium currents. Journal of Neuroscience, 28, 788–797.
Nimmrich, V., Maier, N., Schmitz, D., & Draguhn, A. (2005). Induced sharp wave–ripple complexes in the absence of synaptic inhibition in mouse hippocampal slices. Journal of Physiology, 563, 663–670.
Nishida, K., Yoshimura, M., Isotani, T., Yoshida, T., Kitaura, Y., Saito, A., et al. (2011). Differences in quantitative EEG between frontotemporal dementia and Alzheimer’s disease as revealed by LORETA. Clinical Neurophysiology, 122, 1718–1725.
Osipova, D., Pekkonen, E., & Ahveninen, J. (2006). Enhanced magnetic auditory steady-state response in early Alzheimer’s disease. Clinical Neurophysiology, 117, 1990–1995.
Painold, A., Anderer, P., Holl, A. K., Letmaier, M., Saletu-Zyhlarz, G. M., Saletu, B., & Bonelli, R. M. (2011). EEG low-resolution brain electromagnetic tomography (LORETA) in Huntington’s disease. Journal of Neurology, 258, 840–854.
Palop, J. J., Chin, J., Roberson, E. D., Wang, J., Thwin, M. T., Bien-Ly, N., et al. (2007). Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron, 55, 697–711.
Palop, J. J., & Mucke, L. (2009). Epilepsy and cognitive impairments in Alzheimer Disease. Archives of Neurology, 66, 435–440.
Palop, J. J., & Mucke, L. (2010). Synaptic depression and aberrant excitatory network activity in Alzheimer’s disease: Two faces of the same coin? Neuromolecular. Med, 12, 48–55.
Peña-Ortega, F., & Bernal-Pedraza, R. (2012). Amyloid Beta Peptide slows down sensory-induced hippocampal oscillations. International Journal of Peptides, 2012, 236289.
Persson, J., Lind, J., Larsson, A., Ingvar, M., Sleegers, K., Van Broeckhoven, C., et al. (2008). Altered deactivation in individuals with genetic risk for Alzheimer’s disease. Neuropsychologia, 46, 1679–1687.
Petit, D., Lorrain, D., Gauthier, S., & Montplaisir, J. (1993). Regional spectral analysis of the REM sleep EEG in mild to moderate Alzheimer’s disease. Neurobiology of Aging, 14, 141–145.
Pignatelli, M., Lebreton, F., Cho, Y. H., & Leinekugel, X. (2012). “Ectopic” theta oscillations and interictal activity during slow-wave state in the R6/1 mouse model of Huntington’s disease. Neurobiology of Diseases, 48, 409–417.
Pihlajamäki, M., DePeau, K. M., Blacker, D., & Sperling, R. A. (2008). Impaired medial temporal repetition suppression is related to failure of parietal deactivation in Alzheimer disease. The American Journal of Geriatric Psychiatry, 16, 283–292.
Pijnenburg, Y. A., Strijers, R. L., Made, Y. V., van der Flier, W. M., Scheltens, P., & Stam, C. J. (2008). Investigation of resting-state EEG functional connectivity in frontotemporal lobar degeneration. Clinical Neurophysiology, 119, 1732–1738.
Platt, B., Drever, B., Koss, D., Stoppelkamp, S., Jyoti, A., Plano, A., et al. (2011). Abnormal cognition, sleep, EEG and brain metabolism in a novel knock-in Alzheimer mouse, PLB1. PLoS ONE, 6, e27068.
Polanía, R., Nitsche, M. A., Korman, C., Batsikadze, G., & Paulus, W. (2012). The importance of timing in segregated theta phase-coupling for cognitive performance. Current Biology, 22, 1314–1318.
Ponomareva, N., Klyushnikov, S., Abramycheva, N., Malina, D., Scheglova, N., Fokin, V., et al. (2014). Alpha-theta border EEG abnormalities in preclinical Huntington’s disease. Journal of the Neurological Sciences, 344, 114–120.
Pooler, A. M., Noble, W., & Hanger, D. P. (2014). A role for tau at the synapse in Alzheimer’s disease pathogenesis. Neuropharmacology, 76(Pt A), 1–8.
Prichep, L. S. (2007). Quantitative EEG and electromagnetic brain imaging in aging and in the evolution of dementia. Annals of the New York Academy of Sciences, 1097, 156–167.
Rabinowicz, A. L., Starkstein, S. E., Leiguarda, R. C., & Coleman, A. E. (2000). Transient epileptic amnesia in dementia: A treatable unrecognized cause of episodic amnestic wandering. Alzheimer Disease and Associated Disorders, 14, 231–233.
Ramsden, M., Henderson, Z., & Pearson, H. A. (2002). Modulation of Ca2+ channel currents in primary cultures of rat cortical neurones by amyloid beta protein (1–40) is dependent on solubility status. Brain Research, 956, 254–261.
Ray, P. G., & Jackson, W. J. (1991). Lesions of nucleus basalis alter ChAT activity and EEG in rat frontal neocortex. Electroencephalography and Clinical Neurophysiology, 79, 62–68.
Ray, N. J., Jenkinson, N., Wang, S., Holland, P., Brittain, J. S., Joint, C., et al. (2008). Local field potential beta activity in the subthalamic nucleus of patients with Parkinson’s disease is associated with improvements in bradykinesia after dopamine and deep brain stimulation. Experimental Neurology, 213, 108–113.
Ribary, U., Ioannides, A. A., Singh, K. D., Hasson, R., Bolton, J. P., Lado, F., et al. (1991). Magnetic field tomography of coherent thalamocortical 40-Hz oscillations in humans. Proceedings of the National Academy of Sciences of the United States of America, 88, 11037–11041.
Rodriguez, G., Copello, F., Vitali, P., Perego, G., & Nobili, F. (1999). EEG spectral profile to stage Alzheimer’s disease. Clinical Neurophysiology, 110, 1831–1837.
Romanelli, M. F., Morris, J. C., Ashkin, K., & Coben, L. A. (1990). Advanced Alzheimer’s disease is a risk factor for late-onset seizures. Archives of Neurology, 47, 847–850.
Rombouts, S. A., Barkhof, F., Goekoop, R., Stam, C. J., & Scheltens, P. (2005). Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: An fMRI study. Human Brain Mapping, 26, 231–239.
Rosen, H. J., Hartikainen, K. M., Jagust, W., Kramer, J. H., Reed, B. R., Cummings, J. L., et al. (2002). Utility of clinical criteria in differentiating frontotemporal lobar degeneration (FTLD) from AD. Neurology, 58, 1608–1615.
Sanchez, P. E., Zhu, L., Verret, L., Vossel, K. A., Orr, A. G., Cirrito, J. R., et al. (2012). Levetiracetam suppresses neuronal network dysfunction and reverses synaptic and cognitive deficits in an Alzheimer’s disease model. Proceedings of the National Academy of Sciences of the United States of America, 109, E2895–E2903.
Schlingloff, D., Káli, S., Freund, T. F., Hajos, N., & Gulyás, A. I. (2014). Mechanisms of sharp wave initiation and ripple generation. Journal of Neuroscience, 34, 11385–11398.
Schmitz, D., Fisahn, A., Draguhn, A., Buhl, E. H., Petrasch-Parwez, E., Dermietzel, R., et al. (2001). Axo-axonal coupling. A novel mechanism for ultrafast neuronal communication. Neuron, 31, 831–840.
Schnitzler, A., & Gross, J. (2005). Normal and pathological oscillatory communication in the brain. Nature Reviews Neuroscience, 6, 285–296.
Schnitzler, A., Timmermann, L., & Gross, J. (2006). Physiological and pathological oscillatory networks in the human motor system. Journal of Physiology-Paris, 99, 3–7.
Scott, L., Feng, J., Kiss, T., Needle, E., Atchison, K., Kawabe, T. T., et al. (2012). Age-dependent disruption in hippocampal theta oscillation in amyloid-β overproducing transgenic mice. Neurobiology of Aging, 33, 1481.e13–1481.e23.
Scott, D. F., Heathfield, K. W., Toone, B., & Margerison, J. H. (1972). The EEG in Huntington’s chorea: A clinical and neuropathological study. Journal of Neurology, Neurosurgery and Psychiatry, 35, 97–102.
Seeley, W. W., Crawford, R. K., Zhou, J., Miller, B. L., & Greicius, M. D. (2009). Neurodegenerative diseases target large-scale human brain networks. Neuron, 62, 42–52.
Selkoe, D. J. (2002). Alzheimer’s disease is a synaptic failure. Science, 298, 789–791.
Shah, M., & Catafau, A. M. (2014). Molecular imaging insights into neurodegeneration: Focus on tau PET radiotracers. Journal of Nuclear Medicine, 55, 871–874.
Shankar, G. M., Bloodgood, B. L., Townsend, M., Walsh, D. M., Selkoe, D. J., & Sabatini, B. L. (2007). Natural oligomers of the Alzheimer Amyloid-ß protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signalling pathway. Journal of Neuroscience, 27, 2866–2875.
Siegel, M., Donner, T. H., & Engel, A. K. (2012). Spectral fingerprints of large-scale neuronal interactions. Nature Reviews Neuroscience, 13, 121–134.
Simon, A., Traub, R. D., Vladimirov, N., Jenkins, A., Nicholson, C., Whittaker, R. G., et al. (2014). Gap junction networks can generate both ripple-like and fast ripple-like oscillations. European Journal of Neuroscience, 39, 46–60.
Snyder, E. M., Nong, Y., Almeida, C. G., Paul, S., Moran, T., Choi, E. Y., et al. (2005). Regulation of NMDA receptor trafficking by amyloid-beta. Nature Neuroscience, 8, 1051–1058.
Sperfeld, A. D., Collatz, M. B., Baier, H., Palmbach, M., Storch, A., Schwarz, J., et al. (1999). FTDP-17: An early-onset phenotype with parkinsonism and epileptic seizures caused by a novel mutation. Annals of Neurology, 46, 708–715.
Sperling, R. (2007). Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer’s disease. Annals of the New York Academy of Sciences, 1097, 146–155.
Sperling, R. A., Dickerson, B. C., Pihlajamaki, M., Vannini, P., LaViolette, P. S., Vitolo, O. V., et al. (2010). Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Medicine, 12, 27–43.
Stam, C. J., van Cappellen van Walsum, A. M., Pijnenburg, Y. A., Berendse, H. W., de Munck, J. C., Scheltens, P., & van Dijk, B. W. (2002). Generalized synchronization of MEG recordings in Alzheimer’s Disease: Evidence for involvement of the gamma band. Journal of Clinical Neurophysiology, 19, 562–574.
Stark, E., Roux, L., Eichler, R., Senzai, Y., Royer, S., & Buzsáki, G. (2014). Pyramidal cell–interneuron interactions underlie hippocampal ripple oscillations. Neuron, 83, 467–480.
Steriade, M. (2003). The corticothalamic system in sleep. Front. Biosci, 8, d878–d899.
Stoffers, D., Bosboom, J. L., Deijen, J. B., Wolters, E. C., Berendse, H. W., & Stam, C. J. (2007). Slowing of oscillatory brain activity is a stable characteristic of Parkinson’s disease without dementia. Brain, 130, 1847–1860.
Swaab, D. F., Lucassen, P. J., Salehi, A., Scherder, E. J., van Someren, E. J., & Verwer, R. W. (1998). Reduced neuronal activity and reactivation in Alzheimer’s disease. Progress in Brain Research, 117, 343–377.
Terman, D., Rubin, J. E., Yew, A. C., & Wilson, C. J. (2002). Activity patterns in a model for the subthalamopallidal network of the basal ganglia. Journal of Neuroscience, 22, 2963–2976.
Thevathasan, W., Pogosyan, A., Hyam, J. A., Jenkinson, N., Foltynie, T., Limousin, P., et al. (2012). Alpha oscillations in the pedunculopontine nucleus correlate with gait performance in parkinsonism. Brain, 135, 148–160.
Timmermann, L., & Florin, E. (2012). Parkinson’s disease and pathological oscillatory activity: Is the beta band the bad guy? New lessons learned from low-frequency deep brain stimulation. Experimental Neurology, 233, 123–125.
Traub, R. D., Draguhn, A., Whittington, M. A., Baldeweg, T., Bibbig, A., Buhl, E. H., & Schmitz, D. (2002). Axonal gap junctions between principal neurons: A novel source of network oscillations, and perhaps epileptogenesis. Reviews in the Neurosciences, 13, 1–30.
Traub, R. D., & Whittington, M. A. (2010). Cortical oscillations in health and disease. Oxford, New York: Oxford University Press.
Trottenberg, T., Fogelson, N., Kühn, A. A., Kivi, A., Kupsch, A., Schneider, G. H., & Brown, P. (2006). Subthalamic gamma activity in patients with Parkinson’s disease. Experimental Neurology, 200, 56–65.
van der Hiele, K., Jurgens, C. K., Vein, A. A., Reijntjes, R. H., Witjes-Ané, M. N., Roos, R. A., et al. (2007). Memory activation reveals abnormal EEG in preclinical Huntington’s disease. Movement Disorders, 22, 690–695.
van der Zee, J., Sleegers, K., & Van Broeckhoven, C. (2008). Invited article: the Alzheimer disease-frontotemporal lobar degeneration spectrum. Neurology, 71, 1191–1197.
van Deursen, J. A., Vuurman, E. F., van Kranen-Mastenbroek, V. H., & Riedel, W. J. (2008). Increased EEG gamma band activity in Alzheimer’s disease and mild cognitive impairment. J Neural Transm, 115, 1301–1311.
van Deursen, J. A., Vuurman, E. F., van Kranen-Mastenbroek, V. H., Verhey, F. R., & Riedel, W. J. (2011). 40-Hz steady state response in Alzheimer’s disease and mild cognitive impairment. Neurobiology of Aging, 32, 24–30.
Verret, L., Mann, E. O., Hang, G. B., Barth, A. M., Cobos, I., Ho, K., et al. (2012). Inhibitory interneuron deficit links altered network activity and cognitive dysfunction in Alzheimer model. Cell, 149, 708–721.
Villette, V., Poindessous-Jazat, F., Simon, A., Léna, C., Roullot, E., Bellessort, B., et al. (2010). Decreased rhythmic GABAergic septal activity and memory-associated theta oscillations after hippocampal amyloid-beta pathology in the rat. Journal of Neuroscience, 30, 10991–11003.
Vreugdenhil, M., & Toescu, E. C. (2005). Age-dependent reduction of gamma oscillations in the mouse hippocampus in vitro. Neuroscience, 132, 1151–1157.
Wada, Y., Nanbu, Y., Koshino, Y., Yamaguchi, N., & Hashimoto, T. (1998). Reduced interhemispheric EEG coherence in Alzheimer disease: Analysis during rest and photic stimulation. Alzheimer Disease and Associated Disorders, 12, 175–181.
Walsh, D. M., & Selkoe, D. J. (2007). Aβ oligomers—A decade of discovery. Journal of Neurochemistry, 101, 1172–1184.
Weinberger, M., Hutchison, W. D., Lozano, A. M., Hodaie, M., & Dostrovsky, J. O. (2009). Increased gamma oscillatory activity in the subthalamic nucleus during tremor in Parkinson’s disease patients. Journal of Neurophysiology, 101, 789–802.
Wenk, G. L., Zajaczkowski, W., & Danysz, W. (1997). Neuroprotection of acetylcholinergic basal forebrain neurons by memantine and neurokinin B. Behavioural Brain Research, 83, 129–133.
Whittington, M. A., & Traub, R. D. (2003). Interneuron Diversity series: Inhibitory interneurons and network oscillations in vitro. Trends in Neurosciences, 26, 676–682.
Wu, J., Anwyl, R., & Rowan, M. J. (1995). Beta-Amyloid selectively augments NMDA receptor-mediated synaptic transmission in rat hippocampus. Neuroreport, 6, 2409–2413.
Yamamoto, T., & Hirano, A. (1985). Nucleus raphe dorsalis in Alzheimer’s disease: Neurofibrillary tangles and loss of large neurons. Annals of Neurology, 17, 573–577.
Yener, G. G., Emek-Savaş, D. D., Lizio, R., Çavuşoğlu, B., Carducci, F., Ada, E., et al. (2015). Frontal delta event-related oscillations relate to frontal volume in mild cognitive impairment and healthy controls. International Journal of Psychophysiology. doi:10.1016/j.ijpsycho.2015.02.005.
Yener, G., Güntekin, B., & Başar, E. (2008). Event-related delta oscillatory responses of Alzheimer patients. European Journal of Neurology, 15, 540–547.
Yu, J. T., Chang, R. C., & Tan, L. (2009). Calcium dysregulation in Alzheimer’s disease: From mechanisms to therapeutic opportunities. Progress in Neurobiology, 89, 240–255.
Zempel, H., & Mandelkow, E. M. (2012). Linking amyloid-β and tau: Amyloid-β induced synaptic dysfunction via local wreckage of the neuronal cytoskeleton. Neurodegenerative Diseases, 10, 64–72.
Zhang, S., Han, D., Tan, X., Feng, J., Guo, Y., & Ding, Y. (2012). Diagnostic accuracy of 18 F-FDG and 11 C-PIB-PET for prediction of short-term conversion to Alzheimer’s disease in subjects with mild cognitive impairment. International Journal of Clinical Practice, 66, 185–189.
Acknowledgments
Volker Nimmrich is an employee of AbbVie, and this review was supported by AbbVie. AbbVie also participated in the approval of the review. Andreas Draguhn is faculty at the University of Heidelberg. Nikolai Axmacher is faculty at the University of Bochum and member of the DZNE, Bonn.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nimmrich, V., Draguhn, A. & Axmacher, N. Neuronal Network Oscillations in Neurodegenerative Diseases. Neuromol Med 17, 270–284 (2015). https://doi.org/10.1007/s12017-015-8355-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12017-015-8355-9