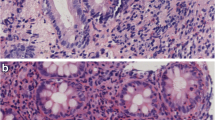
Abstract
Gastrointestinal adverse events are common during oral immunotherapy (OIT) for food allergy and range from immediate IgE-mediated reactions to non-anaphylactic clinical presentations. This review aims to summarize recent findings on non-anaphylactic eosinophil-associated gastrointestinal adverse events during OIT. Two clinical presentations of non-anaphylactic eosinophil-associated gastrointestinal adverse events during OIT are identified, each with a different paradigm for treatment, and distinguished by their time of onset. In the first clinical entity, characterized by its onset early in the course of treatment, patients present with abdominal pain, nausea, and/or vomiting. The symptoms become evident typically within weeks to months of starting OIT. These symptoms, however, are not temporally related to the time of dose administration, as in the case of immediate IgE-mediated anaphylactic reactions. While esophageal biopsies, when performed, can demonstrate eosinophilic esophagitis (EoE), baseline esophageal eosinophilia has also been observed in food allergic patients prior to OIT. A potential non-invasive biomarker, the peripheral absolute eosinophil count (AEC), often rises during these reactions and subsides after dose reduction and subsequent resolution of symptoms. OIT can usually then be resumed, albeit at a slower pace, without a recurrence of symptoms. Risk factors for development of symptoms early during OIT include a high starting dose and a baseline AEC of greater than 600. The second, and much less frequently encountered, non-anaphylactic gastrointestinal adverse event related to OIT, presents months to years after initiating OIT. In this latter group, patients present with the classical clinical symptoms and endoscopic findings of EoE. In contrast to the acute onset group, peripheral eosinophilia is usually not observed in these cases. This OIT-associated EoE has shown good response to standard EoE treatment approaches of proton pump inhibitors or swallowed steroids. Most patients with eosinophil-associated adverse reactions are able to continue OIT and remain desensitized. Treatment approaches depend on the specific subtype of these reactions and relate to the stages of OIT treatment.

Similar content being viewed by others
Abbreviations
- EoE:
-
Eosinophilic esophagitis
- OIT:
-
Oral immunotherapy
- OITIGER:
-
Oral immunotherapy–induced gastrointestinal and eosinophilic responses
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Greenhawt M (2016) Food allergy quality of life and living with food allergy. Curr Opin Allergy Clin Immunol 16(3):284–290
Cummings AJ, Knibb RC, King RM, Lucas JS (2010) The psychological impact of food allergy and food hypersensitivity in children, adolescents and their families: a review. Allergy 65:933–945
Allen CW, Bidarkar MS, vanNunen SA, Campbell DE (2015) Factors impacting parental burden in food allergic children. J Paediatr Child Health 51:696–698
Gupta R, Holdford D, Bilaver L, Dyer A, Holl JL, Meltzer D (2013) The economic impact of childhood food allergy in the United States. JAMA Pediatr 167:1026–1031
Warren CM, Otto AK, Walkner MM, Gupta RS (2016) Quality of life among food allergic patients and their caregivers. Curr Allergy Asthma Rep 16:38
Levy MB, Elizur A, Goldberg MR, Nachshon L, Katz Y (2014) Clinical predictors for favorable outcomes in an oral immunotherapy program for IgE-mediated cow’s milk allergy. Ann Allergy Asthma Immunol 112(1):58-63.e1
Goldberg MR, Nachshon L, Appel MY, Elizur A, Levy MB, Eisenberg E et al (2015) Efficacy of baked milk oral immunotherapy in baked milk-reactive allergic patients. J Allergy Clin Immunol 136(6):1601–1606
Wood RA, Kim JS, Lindblad R, Nadeau K, Henning AK, Dawson P et al (2016) A randomized, double-blind, placebo-controlled study of omalizumab combined with oral immunotherapy for the treatment of cow’s milk allergy. J Allergy Clin Immunol 137(4):1103–1110.e11. https://doi.org/10.1016/j.jaci.2015.10.005. Epub 2015 Nov 12. PMID: 26581915; PMCID: PMC5395304
Vickery BP, Vereda A, Casale TB, Beyer K, Du Toit G, Hourihane JO et al (2018) AR101 Oral immunotherapy for peanut allergy. N Engl J Med 397:1991–2001
Nachshon L, Goldberg MR, Levy MB, Appel MY, Epstein-Rigbi N, Lidholm J et al (2019) Efficacy and safety of sesame oral immunotherapy-a real-world, single-center study. J Allergy Clin Immunol Pract 7(8):2775-2781.e2 PMID: 31150789
Burks AW, Jones SM, Wood RA, Fleischer DM, Sicherer SH, Lindblad RW et al (2012) Consortium of Food Allergy Research (CoFAR). Oral immunotherapy for treatment of egg allergy in children. N Engl J Med 367(3):233–43. PMID: 22808958; PMCID: PMC3424505
Jones SM, Burks AW, Keet C, Vickery BP, Scurlock AM, Wood RA et al (2016) Long-term treatment with egg oral immunotherapy enhances sustained unresponsiveness that persists after cessation of therapy. J Allergy Clin Immunol 137(4):1117–1127.e10
Kim EH, Perry TT, Wood RA, Leung DYM, Berin MC, Burks AW et al (2020) Consortium for Food Allergy Research (CoFAR). Induction of sustained unresponsiveness after egg oral immunotherapy compared to baked egg therapy in children with egg allergy. J Allergy Clin Immunol 146(4):851–862.e10
Elizur A, Appel MY, Nachshon L, Levy MB, Epstein-Rigbi N, Golobov K et al (2018) NUT Co Reactivity - ACquiring Knowledge for Elimination Recommendations (NUT CRACKER) study. Allergy 73(3):593–601. https://doi.org/10.1111/all.13353. Epub 2017 Dec 5 PMID: 29127694
Elizur A, Appel MY, Nachshon L, Levy MB, Epstein-Rigbi N, Pontoppidan B et al (2019) Walnut oral immunotherapy for desensitisation of walnut and additional treenut allergies (Nut CRACKER): a single-centre, prospective cohort study. Lancet Child Adolesc Health 3(5):312–321. https://doi.org/10.1016/S2352-4642(19)30029-X. Epub 2019 Mar 27. Erratum in: Lancet Child Adolesc Health 3(7):e10. PMID: 30926371
Elizur A, Appel MY, Nachshon L, Levy MB, Epstein-Rigbi N, Koren Y et al (2022) Cashew oral immunotherapy for desensitizing cashew-pistachio allergy (NUT CRACKER study). Allergy. https://doi.org/10.1111/all.15212. Epub ahead of print. PMID: 35000223
Pajno GB, Fernandez-Rivas M, Arasi S, Roberts G, Akdis CA, Alvaro- Lozano M et al (2018) EAACI guidelines on allergen immunotherapy: IgE-mediated food allergy. Allergy 73:799–815
Bégin P, Chan ES, Kim H, Wagner M, Cellier MS, Favron-Godbout C et al (2020) CSACI guidelines for the ethical, evidence-based and patient-oriented clinical practice of oral immunotherapy in IgE-mediated food allergy. Allergy Asthma Clin Immunol 16:20
Martorell A, Alonso E, Echeverría L, Escudero C, García-Rodríguez R, Blasco C et al (2017) Immunotherapy egg and milk Spanish guide (ITEMS guide). Part I: cow milk and Egg oral immunotherapy: introduction, methodology, rationale, current state, indications, contraindications, and oral immunotherapy build-up phase. J Investig Allergol Clin Immunol 27(4):225–237
Pajno GB, Bernardini R, Peroni D, Arasi S, Martelli A, Landi M et al (2017) Allergen-specific Immunotherapy panel of the Italian Society of Pediatric Allergy and Immunology (SIAIP). Clinical practice recommendations for allergen-specific immunotherapy in children: the Italian consensus report. Ital J Pediatr 43(1):13
Ebisawa M, Ito K, Fujisawa T (2020) Committee for Japanese Pediatric Guideline for Food Allergy, The Japanese Society of Pediatric Allergy and Clinical Immunology; Japanese Society of Allergology. Japanese guidelines for food allergy 2020. Allergol Int 69(3):370–386
Nachshon L, Goldberg MR, Katz Y, Levy MB, Elizur A (2018) Long-term outcome of peanut oral immunotherapy – real life experience. Pediatr Allergy Immunol 29(5):519–526
Epstein-Rigbi N, Goldberg MR, Levy MB, Nachshon L, Elizur A (2019) Quality of life of food-allergic patients before, during, and after oral immunotherapy. J Allergy Clin Immunol Pract 7(2):429-436.e2. https://doi.org/10.1016/j.jaip.2018.06.016. Epub 2018 Jul 7 PMID: 30129441
Epstein-Rigbi N, Goldberg MR, Levy MB, Nachshon L, Elizur A (2020) Quality of life of children aged 8–12 years undergoing food allergy oral immunotherapy: child and parent perspective. Allergy 75(10):2623–2632 PMID: 32350869
Otani IM et al (2014) Multiple-allergen oral immunotherapy improves quality of life in caregivers of food-allergic pediatric subjects. Allergy Asthma Clin Immunol 10:25
Chu DK, Wood RA, French S, Fiocchi A, Jordana M, Waserman S et al (2019) Oral immunotherapy for peanut allergy (PACE): a systematic review and meta-analysis of efficacy and safety. Lancet 393(10187):2222–2232. https://doi.org/10.1016/S0140-6736(19)30420-9. Epub 2019 Apr 25. Erratum in: Lancet. 2019;393(10184):1936. PMID: 31030987
Eiwegger T, Anagnostou K, Arasi S, Bégin P, Ben-Shoshan M, Beyer K et al (2019) ICER report for peanut OIT comes up short. Ann Allergy Asthma Immunol 123(5):430–432. https://doi.org/10.1016/j.anai.2019.09.001. Epub 2019 Sep 9 PMID: 31513908
Nachshon L, Goldberg MR, Elizur A, Levy MB, Schwartz N, Katz Y (2015) A web site-based reporting system for monitoring home treatment during oral immunotherapy for food allergy. Ann Allergy Asthma Immunol 114(6):510–515. https://doi.org/10.1016/j.anai.2015.04.007. Epub 2015 May 1 PMID: 25940735
Petroni D, Spergel JM (2018) Eosinophilic esophagitis and symptoms possibly related to eosinophilic esophagitis in oral immunotherapy. Ann Allergy Asthma Immunol 120(3):237–240.e4..2017.11.016
Nachshon L, Schwartz N, Tsviban L, Levy MB, Goldberg MR, Epstein-Rigby N et al (2021) Patient characteristics and risk factors for home epinephrine-treated reactions during oral immunotherapy for food allergy. J Allergy Clin Immunol Pract 9(1):185-192.e3 Epub 2020 Aug 1 PMID: 32750430
Nachshon L, Levy MB, Goldberg MR, Epstein-Rigbi N, Schwartz N, Katz Y et al (2022) Triggers for home epinephrine-treated reactions during oral immunotherapy for food allergy. J Allergy Clin Immunol Pract S2213–2198(21):01450–01451 PMID: 34982978
Shaker MS, Wallace DV, Golden DBK, Oppenheimer J, Bernstein JA, Campbell RL et al (2020) Anaphylaxis-a 2020 practice parameter update, systematic review, and Grading of Recommendations, Assessment, Development and Evaluation (GRADE) analysis. J Allergy Clin Immunol 145(4):1082–1123
Muraro A, Werfel T, Hoffmann-Sommergruber K, Roberts G, Beyer K, Bindslev-Jensen C et al (2014) EAACI food allergy and anaphylaxis guidelines group. EAACI food allergy and anaphylaxis guidelines: diagnosis and management of food allergy. Allergy 69(8):1008–25. https://doi.org/10.1111/all.12429
Cardona V, Ansotegui IJ, Ebisawa M, El-Gamal Y, Fernandez Rivas M, Fineman S et al (2020) World allergy organization anaphylaxis guidance 2020. World Allergy Organ J 13(10):100472. https://doi.org/10.1016/j.waojou.2020.100472. PMID: 33204386; PMCID: PMC7607509
Young VB (2017) Effective management of pain and anxiety for the pediatric patient in the emergency department. Crit Care Nurs Clin North Am 29(2):205–216. https://doi.org/10.1016/j.cnc.2017.01.007. Epub 2017 Mar 18 PMID: 28460701
Lemos L, Assis HC, Alves JL, Reis DS, Campos Canesso MC et al (2022) Neuroimmune circuits involved in β-lactoglobulin-induced food allergy. Brain Behav Immun Health 21(23):100471 PMID: 35668724
Florsheim EB, Bachtel ND, Cullen J, Lima BGC, Godazgar M, Zhang C et al (2023) Immune sensing of food allergens promotes aversive behaviour. bioRxiv [Preprint]. 2023 Jan 20:2023.01.19.524823. https://doi.org/10.1101/2023.01.19.524823. Update in: Nature. PMID: 36712030; PMCID: PMC9882358
Goldberg MR, Elizur A, Nachshon L, Appel MY, Levy MB, Golobov K et al (2017) Oral immunotherapy-induced gastrointestinal symptoms and peripheral blood eosinophil responses. J Allergy Clin Immunol 139(4):1388-1390.e4. https://doi.org/10.1016/j.jaci.2016.09.053. Epub 2016 Nov 29 PMID: 27913305
• Wright BL, Fernandez-Becker NQ, Kambham N, Purington N, Cao S, Tupa D et al (2021) Gastrointestinal eosinophil responses in a longitudinal, randomized trial of peanut oral immunotherapy. Clin Gastroenterol Hepatol 19(6):1151–1159.e14. https://doi.org/10.1016/j.cgh.2020.05.019. PMID: 32434067; PMCID: PMC8445108. Baseline esophageal eosinophilia was noted in some subjects that increased during OIT, albeit it did not correlate with symptoms.
Underwood B, Troutman TD, Schwartz JT (2023) Breaking down the complex pathophysiology of eosinophilic esophagitis. Ann Allergy Asthma Immunol 130(1):28–39. https://doi.org/10.1016/j.anai.2022.10.026. Epub 2022 Nov 6. PMID: 36351516
• Goldberg MR, Nachshon L, Levy MB, Elizur A, Katz Y (2020) Risk Factors and Treatment Outcomes for Oral Immunotherapy-Induced Gastrointestinal Symptoms and Eosinophilic Responses (OITIGER). J Allergy Clin Immunol Pract 8(1):125–131. https://doi.org/10.1016/j.jaip.2019.07.034. Epub 2019 Aug 2. PMID: 31382040. Treatment by decreasing dose and/or rate of allergen progression during OIT, alleviated non-anaphylactic symptoms and blood eosinophils normalized to baseline.
Stein ML, Levy MM, Goldberg MR et al (2012) Classification, prevalence and outcomes of non-IgE mediated reactions to oral food immunotherapy. J Allergy Clin Immunol 129:AB29
Wasserman RL, Hague AR, Pence DM et al (2019) Real-world experience with peanut oral immunotherapy: lessons learned From 270 patients. J Allergy Clin Immunol Pract 7(2):418-426.e4. https://doi.org/10.1016/j.jaip.2018.05.023
•• Epstein-Rigbi N, Elizur A, Levy MB, Nachshon L, Koren Y, Shalem Z et al (2023) Treatment of oral immunotherapy-associated eosinophilic esophagitis. J Allergy Clin Immunol Pract 11(4):1303–1305.e2. https://doi.org/10.1016/j.jaip.2022.11.010. OIT-associated EoE can be successfully treated with PPI and/or SWCS without having to discontinue treatment.
Echeverria-Zudaire LA, Fernandez-Fernandez S, Rayo-Fernandez A, Munoz-Archidona C, Checa-Rodriguez R (2016) Primary eosinophilic gastrointestinal disorders in children who have received food oral immunotherapy. Allergol Immunopathol (Madr) 44:531–536
Capucilli P, Hill DA (2019) Allergic comorbidity in eosinophilic esophagitis: mechanistic relevance and clinical implications. Clin Rev Allergy Immunol 57(1):111–127. https://doi.org/10.1007/s12016-019-08733-0. PMID: 30903437; PMCID: PMC6626558
Hamant L, Freeman C, Garg S, Wright BL, Schroeder S (2021) Eosinophilic esophagitis may persist after discontinuation of oral immunotherapy. Ann Allergy Asthma Immunol 126(3):299–302. https://doi.org/10.1016/j.anai.2020.12.007. Epub 2020 Dec 17. PMID: 33340664; PMCID: PMC7897277
Lucendo AJ, Arias A, Tenias JM (2014) Relation between eosinophilic esophagitis and oral immunotherapy for food allergy: a systematic review with meta-analysis. Ann Allergy Asthma Immunol 113:624–629 ([PubMed: 25216976])
Sanchez-Garcia S, Rodriguez Del Rio P, Escudero C, Martinez-Gomez MJ, Ibanez MD (2012) Possible eosinophilic esophagitis induced by milk oral immunotherapy. J Allergy Clin Immunol 129:1155–1157 ([PubMed: 22236725])
Burk CM, Dellon ES, Steele PH, Virkud YV, Kulis M, Burks AW, Vickery BP (2017) Eosinophilic esophagitis during peanut oral immunotherapy with omalizumab. J Allergy Clin Immunol Pract 5:489–501 ([PubMed: 27843067])
Fuentes-Aparicio V, Alvarez-Perea A, Infante S, Zapatero L, D’Oleo A, Alonso-Lebrero E (2013) Specific oral tolerance induction in paediatric patients with persistent egg allergy. Allergol Immunopathol (Madr) 41:143–150
Nowak-Wegrzyn AH, Strong BD, Ananos D, Sampson HA (2013) Long term follow up of children who incorporated extensively heated (baked milk) in the diet. J Allergy Clin Immunol 133:AB107
Kapel RC, Miller JK, Torres C, Aksoy S, Lash R, Katzka DA (2008) Eosinophilic esophagitis: a prevalent disease in the United States that affects all age groups. Gastroenterology 134(5):1316–1321
Wright BL, Fernandez-Becker NQ, Kambham N et al (2018) Baseline gastrointestinal eosinophilia is common in oral immunotherapy subjects With IgE-mediated peanut allergy. Front Immunol 9:2624. Published 2018 Nov 22. https://doi.org/10.3389/fimmu.2018.02624
Barbosa AC, Castro FM, Meireles PR, Arruda LK, Cardoso SR, Kalil J et al (2018) Eosinophilic esophagitis: latent disease in patients with anaphylactic reaction to cow’s milk. J Allergy Clin Immunol Pract 6(2):451–456.e1
Avinashi V, Al Yarubi Z, Soller L, Lam G, Chan ES (2021) Oral peanut immunotherapy acutely unmasking eosinophilic esophagitis with an esophageal stricture. Ann Allergy Asthma Immunol 127(6):691–692. https://doi.org/10.1016/j.anai.2021.09.001. Epub 2021 Sep 10 PMID: 34517129
Ackerman SJ, Kagalwalla AF, Hirano I, Gonsalves N, Katcher PM, Gupta S (2019) One-hour esophageal string test: a nonendoscopic minimally invasive test that accurately detects disease activity in eosinophilic esophagitis. Am J Gastroenterol 114(10):1614–1625. https://doi.org/10.14309/ajg.0000000000000371. PMID:31567192
Hirano I, Chan ES, Rank MA, Sharaf RN, Stollman NH, Stukus DR et al (2020) AGA Institute Clinical Guidelines Committee; Joint Task Force on Allergy-Immunology Practice Parameters. AGA institute and the joint task force on allergy-immunology practice parameters clinical guidelines for the management of eosinophilic esophagitis. Gastroenterology 158(6):1776–1786. https://doi.org/10.1053/j.gastro.2020.02.038. PMID: 32359562
Dellon ES, Liacouras CA, Molina-Infante J, Furuta GT, Spergel JM, Zevit N et al (2018) Updated international consensus diagnostic criteria for eosinophilic esophagitis: proceedings of the AGREE conference. Gastroenterology 155(4):1022–1033.e10. PMID: 30009819; PMCID: PMC6174113
Shalem T, Cohen DL, Epstein-Rigbi N, Elizur A, Eindor-Abarbanel A, Broide E, Richter V (2023) Proton pump inhibitors in eosinophilic esophagitis (EoE) related to oral immunotherapy: is it as effective as in other EoE? Eur J Pediatr. https://doi.org/10.1007/s00431-023-05228-1. Epub ahead of print. PMID: 37750913
Morales-Cabeza C, Infante S, Cabrera-Freitag P, Fuentes-Aparicio V, Zubeldia JM, Álvarez-Perea A (2023) Oral immunotherapy and risk of eosinophilic esophagitis in children: 15 years’ experience. J Pediatr Gastroenterol Nutr 76(1):53–58. https://doi.org/10.1097/MPG.0000000000003631. Epub 2022 Oct 4 PMID: 36190840
Wechsler JB, Ackerman SJ, Chehade M, Amsden K, Riffle ME, Wang MY et al (2021) Noninvasive biomarkers identify eosinophilic esophagitis: a prospective longitudinal study in children. Allergy 76(12):3755–3765. https://doi.org/10.1111/all.14874. PMID: 33905577
Liacouras CA, Spergel J, Gober LM (2014) Eosinophilic esophagitis: clinical presentation in children. Gastroenterol Clin North Am 43(2):219–229. https://doi.org/10.1016/j.gtc.2014.02.012. PMID: 24813511
O’Shea KM, Aceves SS, Dellon ES et al (2018) Pathophysiology of eosinophilic esophagitis. Gastroenterology 154(2):333–345
Stein ML, Collins MH, Villanueva JM, Kushner JP, Putnam PE, Buckmeier BK et al (2006) Anti-IL-5 (mepolizumab) therapy for eosinophilic esophagitis. J Allergy Clin Immunol 118(6):1312–1319. https://doi.org/10.1016/j.jaci.2006.09.007. PMID: 17157662
Hirano I, Dellon ES, Hamilton JD, Collins MH, Peterson K, Chehade M et al (2020) Efficacy of dupilumab in a phase 2 randomized trial of adults with active eosinophilic esophagitis. Gastroenterology 158(1):111-122.e10 PMID: 31593702
MacGinnitie AJ, Rachid R, Gragg H, Little SV, Lakin P, Cianferoni A et al (2017) Omalizumab facilitates rapid oral desensitization for peanut allergy. J Allergy Clin Immunol 139(3):873-881.e8
• Nilsson C, Scurlock AM, Dellon ES, Brostoff JM, Pham T, Ryan R et al (2021) Onset of eosinophilic esophagitis during a clinical trial program of oral immunotherapy for peanut allergy. J Allergy Clin Immunol Pract 9(12):4496–4501. https://doi.org/10.1016/j.jaip.2021.07.048. Includes data from 2 phase 2 and three phase 3 trials during peanut OIT.
García Vega M, Fernández-Fernández S, Echeverría Zudaire L, Bracamonte Bermejo T, Cano Del Águila B et al (2022) Long-term medical treatment efficacy in patients with eosinophilic oesophagitis and oral food immunotherapy. Clin Exp Allergy 52(12):1440–1443. https://doi.org/10.1111/cea.14219. Epub 2022 Aug 29 PMID: 35993507
García Rodríguez R, Morano Lozano L, Extremera Ortega A, Gonzalez Jiménez OM, Borja Segade J, Gomez TE (2020) Eosinophilic esophagitis is probably a comorbid condition in egg-allergic patients undergoing egg oral immunotherapy. J Investig Allergol Clin Immunol 30(1):60–61. https://doi.org/10.18176/jiaci.0438. PMID: 32077855
Mori F, Cianferoni A, Brambilla A, Barni S, Sarti L, Pucci N et al (2017) Side effects and their impact on the success of milk oral immunotherapy (OIT) in children. Int J Immunopathol Pharmacol 30(2):182–187. https://doi.org/10.1177/0394632017697986. Epub 2017 Mar 14. PMID: 28466667; PMCID: PMC5806791
Narisety SD, Skripak JM, Steele P, Hamilton RG, Matsui EC, Burks AW et al (2009) Open-label maintenance after milk oral immunotherapy for IgE-mediated cow’s milk allergy. J Allergy Clin Immunol 124(3):610–2. https://doi.org/10.1016/j.jaci.2009.06.025. Epub 2009 Aug 8. PMID: 19665770; PMCID: PMC2739248
Gómez Torrijos E, Mendez Díaz Y, Moreno Lozano L, Extremera Ortega AM, Borja Segade J, Feo Brito JF et al (2017) Frequency and course of eosinophilic esophagitis during oral immunotherapy for cow’s milk allergy in a series of 57 children. J Investig Allergol Clin Immunol 27(2):132–133. https://doi.org/10.18176/jiaci.0130. PMID: 28398201
Jin H, Trogen B, Nowak-Wegrzyn A (2020) Eosinophilic esophagitis as a complication of food oral immunotherapy. Curr Opin Allergy Clin Immunol 20(6):616–623. https://doi.org/10.1097/ACI.0000000000000688. PMID: 32889961
Virkud YV, Burks AW, Steele PH, Edwards LJ, Berglund JP, Jones SM et al (2017) Novel baseline predictors of adverse events during oral immunotherapy in children with peanut allergy. J Allergy Clin Immunol 139(3):882–888.e5. https://doi.org/10.1016/j.jaci.2016.07.030. Epub 2016 Sep 5. PMID: 27609653; PMCID: PMC5337444
Funding
Dr. Goldberg is funded by a Kamea grant from the Ministry of Health, Israel.
Author information
Authors and Affiliations
Contributions
MG interpreted the data, and conceived and drafted the manuscript. N. ER. aided in acquiring and interpreting the data. AE interpreted the data and drafted the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human or animal subjects performed by any of the authors.
Competing Interests
There authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Goldberg, M.R., Epstein-Rigbi, N. & Elizur, A. Eosinophil-Associated Gastrointestinal Manifestations During OIT. Clinic Rev Allerg Immunol 65, 365–376 (2023). https://doi.org/10.1007/s12016-023-08974-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-023-08974-0