Abstract
Biologic TNFα inhibitors are a mainstay treatment option for patients with rheumatoid arthritis (RA) refractory to other treatment options. However, many patients either do not respond or relapse after initially responding to these agents. This study was carried out to identify biomarkers that can distinguish responder from non-responder patients before the initiation of treatment. The level of cytokines in plasma and those produced by ex vivo T cells, B cells and monocytes in 97 RA patients treated with biologic TNFα inhibitors was measured before treatment and after 1 and 3 months of treatment by multiplex analyses. The frequency of T cell subsets and intracellular cytokines were determined by flow cytometry. The results reveal that pre-treatment, T cells from patients who went on to respond to treatment with biologic anti-TNFα agents produced significantly more GM-CSF than non-responder patients. Furthermore, immune cells from responder patients produced higher levels of IL-1β, TNFα and IL-6. Cytokine profiling in the blood of patients confirmed the association between high levels of GM-CSF and responsiveness to biologic anti-TNFα agents. Thus, high blood levels of GM-CSF pre-treatment had a positive predictive value of 87.5% (61.6 to 98.5% at 95% CI) in treated RA patients. The study also shows that cells from most anti-TNFα responder patients in the current cohort produced higher levels of GM-CSF and TNFα pre-treatment than non-responder patients. Findings from the current study and our previous observations that non-responsiveness to anti-TNFα is associated with high IL-17 levels suggest that the disease in responder and non-responder RA patients is likely to be driven/sustained by different inflammatory pathways. The use of biomarker signatures of distinct pro-inflammatory pathways could lead to evidence-based prescription of the most appropriate biological therapies for different RA patients.
Similar content being viewed by others
Introduction
Rheumatoid arthritis (RA) is a debilitating disease characterised by autoimmunity and the production of high levels of pro-inflammatory cytokines resulting in joint inflammation, the production of matrix-degrading enzymes and cartilage and bone destruction [1]. Epidemiological and genetic studies indicate that susceptibility to RA is associated with HLA-DR alleles and genes encoding cytokines and proteins that regulate T cell responses, highlighting the importance of T cells in disease pathogenesis [2].
Knowledge of the principal pro-inflammatory mediators that cause synovial inflammation in RA led to the discovery and use of biological TNFα inhibitors as effective therapies for treating patients [3]. Most treated patients undergo remission, especially if anti-TNFα is administered soon after diagnosis [4, 5]. However, a significant proportion of patients established disease either do not respond to the treatment or relapse after an initial response, suggesting that the disease in these patients is not driven by TNFα. Exactly why some RA patients do not respond to TNFα inhibitors while others relapse after an initial response remains unclear. One possible explanation for the inconsistent or incomplete response of RA patients to anti-TNFα is that TNFα, perhaps paradoxically, also has regulatory functions. For example, although there is good evidence that the activity of regulatory T cells improves following treatment with anti-TNFα [6,7,8], there is also evidence that TNFα modulates TCR-mediated signalling [9, 10]. An alternative explanation may be that chronic inflammation and disease processes in non-responder patients are not wholly driven by TNFα. In this regard, a number of laboratories including ours have revealed that T cells from non-responder RA patients produce high levels of IL-17 and have a high frequency of circulating Th17 cells [11, 12]. Moreover, our studies have revealed that treating patients with TNFα inihibitors increases the frequency of Th17 cells and IL-17 production, possibly through increasing IL-12/IL-23 p40 production by monocytes/macrophages [12]. Although the molecular basis and the physiological and therapeutic implications remain to be determined, these findings indicate that TNFα/TNFα blockade have significant impacts on the function and frequency of T cell subsets [13]. This suggestion is consistent with the association between T cell responses and signalling generated by TNFα through its two receptors in T cells [reviewed in [14]].
The available evidence suggests that mechanisms driving inflammation in RA could be different in different patients as indicated by variations in the response of individual patients to treatment with anti-TNFα [7, 11, 12]. Therefore, a major challenge has been in the identification of alternative pathways that drive disease in non-responder patients before treatment with TNFα inhibitors starts to enable better, evidence-based therapeutic use of these biological agents. A large number of studies from many laboratories explored the predictive value of many biomarkers including cytokines produced by peripheral blood mononuclear cells or the transcriptome prior to treatment (summarised in Table 1). In the current study, we assess the phenotype and response of T cell subsets in RA patients prescribed anti-TNFα before treatment in relation to the patient’s subsequent response/lack of response to treatment. The results provide evidence for relationships between response/lack of response to anti-TNFα and the frequency of T cell subsets and patterns of plasma levels of pro-inflammatory cytokines prior to treatment.
Patients and Methods
Patients
Patients were recruited from rheumatology clinics at Barts Health NHS, the Royal Free London NHS Foundation and Imperial College Healthcare Trusts with patients’ informed consent. The study was approved by the Ethical Committee (06/Q0605/8; NRES Committee London—City & East) and conducted in compliance with the Declaration of Helsinki 2013. The patients were prescribed anti-TNFα based on NICE guidelines which include failure to respond to treatment with two disease-modifying agents one of which is methotrexate and with a DAS28 score >5.1. Patients were treated with adalimumab, certolizumab pegol, golimumab or etanercept in combination with methotrexate. Patients’ response to treatment was based on the European League Against Rheumatism (EULAR) response criteria [28]; patients were considered responders when their DAS28 decreased by >1.2 to ≤3.2 after 3 months of treatment. All other treated patients were considered non-responders. Immediately prior to treatment and after 1 and 3 months of treatment, 25 mL blood was drawn and B and T cells and monocytes enriched and phenotypically characterised by FACS stimulated and cultured. Plasma were separated and stored at −80 °C until tested.
Reagents
PerCP-Cy5.5-conjugated anti-TNFα, eFluor® 660 anti-GM-CSF, PE-Cy7 anti-IFNγ, PE anti-IL-17A, FITC-anti-CD45RA, PE-anti-CD45RO, eFluor® 450-anti-CD161 and unconjugated anti-CD28 antibodies (Abs) were from eBioscience. Brilliant Violet 605-anti-CCR6 and APC-anti-IL-10 Abs were purchased from BioLegend. RosetteSep negative-enrichment kits for human T cells, B cells and monocytes were from StemCell Technologies. Ficoll-Paque was from GE Healthcare, RPMI from Lonza and foetal bovine serum from Life Technologies. Lipopolysaccharide (E. coli, serotype 0111:B4), ionomycin and PMA were from Sigma-Aldrich and Golgiplug™ was from BD Bioscience and Leucoperm from AbD Serotec.
Cell Enrichment and Analysis
Blood T and B cells and monocytes were enriched by negative selection with RosetteSep kits using mononuclear cells separated by Ficoll-Paque centrifugation. The purity of enriched T cells was consistently ≥95% while for monocyte and B cells purity was 86–98%. The cells were suspended in RPMI containing 10% FCS and antibiotics and analysed by FACS or stimulated in vitro. T cells were stimulated with 10 μg/mL anti-CD3 mAb (clone OKT3) pre-coated onto sterile tissue culture plates and 10 μg/mL anti-CD28 (clone 28.2) in the medium. B cells were stimulated with 10 μg/mL goat F(ab’)2 anti-human IgM coated onto wells of the plates and 10 μg/mL antibody to CD40 (clone G28-5) in the medium. Monocytes were stimulated with 1 μg/mL LPS (E. coli, serotype 0111:B4). Supernatants were collected after 48 h, debris removed by centrifugation and cytokines quantified using Meso Scale Discovery (MSD) multiplex kits or ELISA. To verify that levels of TNFα produced by T cells and monocytes were representative of what the cells produce independent of the stimulation protocol, T cells and monocytes from three healthy controls (HCs) were also stimulated independently for 48 h with 0.1 μg/mL PMA and ionomycin. In addition, T cells were stimulated with 0.1 μg/mL PMA and ionomycin in the presence of 1 μl/mL Golgiplug™ (BD Bioscience) overnight to determine the level of intracellular cytokines. The cells were stained for CD45RA, CD45RO, CCR6 and CD161 by incubation with conjugated monoclonal antibodies (mAbs) for 20 min at 4 °C, permeabilized using Leucoperm (AbD Serotec) and stained for intracellular cytokines with labelled mAbs for IL-17, INFγ, TNFα, GM-CSF and IL-10. The cells were fixed with 0.5% p-formaldehyde in PBS and analysed. FACS was performed using a BD-LSR Fortessa X20 (BD Biosciences) and cell populations were gated and assessed using FacsDiva 6.0 software (BD Bioscience).
Cytokine Measurements
For quantification of cytokines, multiplex MSD kits or sandwich ELISA was used. Conditions for cytokine measurements including determining optimal dilutions of different clinical samples were carried out prior to the measurements as reported [12]. Level of the following cytokines/chemokines was determined in culture supernatants of ex vivo stimulated T and B cells and monocytes and in plasma: TNFα, IL-17, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, IL-20, IL-22, IL-23p40, GM-CSF, IFNγ and MCP-1.
Statistical Analyses
Comparison of patterns of cytokine production was with the Multi Experiment Viewer software (Dana Faber Cancer Institute, Boston, MA). Hierarchal clustering was with Pearson’s correlation with complete linkage. Where indicated, statistical analyses were carried out using the GraphPad Prism 6 software. The Mann-Whitney U test, Wilcoxon matched-pairs, signed rank test, Fisher’s exact test or the Chi2 test were used for the analysis of differences between or within groups, as appropriate. Positive and negative predictive values were calculated using the online program https://www.medcalc.net/tests/diagnostic_test.php.
Results
Patient Response to Therapy
Ninety-seven RA patients prescribed anti-TNFα were recruited to the study (Table 2) and their clinical samples used in multiple experiments described in this report. The patients were treated with one of the following four anti-TNFα agents: adalimumab, certolizumab pegol, golimumab or etanercept in combination with methotrexate. No notable or consistent differences in cytokine production by enriched cells were seen between patients receiving different anti-TNFα agents. Assessment of patients’ response to anti-TNFα was based on the EULAR response criteria at 3 months after treatment. Seventy-six patients (78%) responded to the treatment, slightly higher than previously reported [6,7,8, 11].
Distinct Pro-inflammatory T Cell Cytokine Profiles Predict Responsiveness or Lack of Responsiveness to Treatment with Anti-TNFα
In a previous study, we determined that prior to treatment with anti-TNFα, non-responder RA patients had high frequencies of IL-17+ T cells and, when their T cells stimulated in vitro, the cells produced significantly higher levels of IL-17 than responder patients [12]. The current study compared the profile of cytokines produced by enriched T and B cells and monocytes in responder and non-responder patients prior to and after the start of treatment to establish whether distinct inflammatory pathways associate with the disease in responder as compared with non-responder patients. Levels of TNFα, IL-17, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12p70, IL-13, IL-20, IL-22, IL-23p19, GM-CSF, IFNγ and MCP-1 either in culture supernatants of the stimulated T and B cells and monocytes or detected in plasma collected from most of the patients at the time of sampling were measured. For clarity of our observations, only data for cytokine measurements that consistently showed significant differences between responder and non-responder patients are presented and discussed in detail in this report.
Monocytes are generally believed to be a main source of TNFα in joints of RA patients although there is evidence that the cytokine is also produced by T cells [29,30,31,32]. The current study confirmed that monocytes, T and B cells all produce TNFα when stimulated in vitro (Fig. 1a). However, T cells stimulated with anti-CD3/CD28 mAbs produced almost 45-fold the level of TNFα produced by LPS-stimulated monocytes (P < 0.0001; Fig. 1a and Table 3). To exclude the possibility that the higher levels of TNFα produced by T cells were merely due to the activation protocol and not capacity of the cells themselves, both lineage cells were independently stimulated with PMA/ionomycin in parallel experiments. The mean and SEM level of TNFα produced by T cells stimulated with PMA/ionomycin was 5.9 ± 4.3 ng/mL, >10-fold higher than what monocytes produced with PMA/ionomycin at 0.5.8 ± 0.4 ng/mL (P < 0.05; Fig. 1b). Before treatment, T cells from responder patients stimulated with anti-CD3/CD28 mAbs tended to produce more TNFα than non-responder patients (P = 0.20; Fig. 1a and Table 3).
Cytokine production by T cells, B cells and monocytes in rheumatoid arthritis patients prior to and after treatment with anti-TNFα. T cells, B cells and monocytes were enriched by negative selection from the blood of RA patients immediately before treatment with anti-TNFα agents and then after 1 and 3 months. The cells were stimulated for 48 h and the level of cytokines produced determined using MSD multiplex kits. Levels of 16 cytokines were determined but only results of cytokines with notable differences are presented: a, b Data on TNFα; c IL-1β and d GM-CSF. a TNFα produced by T cells stimulated with anti-CD3/anti-CD28 mAbs, B cells stimulated with anti-IgM/anti-CD40 and monocytes stimulated with LPS. b Mean ± standard error of the mean (SEM) for TNFα produced by T cells and monocytes from 3 healthy controls and stimulated for 48 h with PMA and ionomycin. c, d Levels of IL-1β and GM-CSF produced by T and B cells and monocytes activated and cultured as described in a. e Data on GM-CSF production by T cells as described for a before treatment (0 months) and after 1 and then 3 months. f Data on the frequency of GM-CSF+ T cells before treatment and after 1 and 3 months in responder and non-responder RA patients. Enriched T cells were stimulated with PMA/ionomycin for 16 h and stained for intracellular GM-CSF. g IL-6 production by T cells and h CCL2 production by monocytes. The data before and after treatment are for 67 patients. Differences between responders and non-responders were assessed using two-tailed Mann Whitney U test. Difference at different time points in the same group was assessed using Wilcoxon matched-pairs signed rank test. Single asterisk indicates P < 0.05; double asterisk indicates P < 0.01
T cells from responder patients produced sevenfold higher levels of IL-1β than T cells from non-responders (Fig. 1c and Table 3). However, monocytes produced higher levels of IL-1β than T cells but there was no difference in IL-1β production by monocytes from responder and non-responder patients. As expected, B cells produced less IL-1β than monocytes but there was a trend for higher levels in responder patients (Fig. 1c and Table 3).
T cells produced significantly higher levels of GM-CSF compared with monocytes and B cells (P < 0.0001; Fig. 1d and Table 3). Importantly, GM-CSF levels produced by T cells were significantly higher in responder compared with non-responder patients before treatment (P < 0.05; Fig. 1d,e and Table 3). There was a significant increase in GM-CSF production by T cells in responder patients after 1 month of treatment but declined at 3 months (P < 0.05, Fig. 1e). Consistent with higher GM-CSF levels produced by T cells from responder patients, there were also more GM-CSF+ T cells in the blood of responder patients before (3.0 ± 2.0 vs. 1.4 ± 1.4%) and after 1 month (3.1 ± 2.0 vs. 1.4 ± 1.0%) of treatment (Fig. 1f).
Monocytes, T and B cells produced IL-6 with monocytes producing 10-fold higher levels than T and B cells (P < 0.0001; Table 3). T cells from responder patients produced >3-fold IL-6 levels more than T cells from non-responder patients before treatment (P = 0.27, Fig. 1g and Table 3).
MCP1 was produced almost exclusively by monocytes with 1.8-fold higher levels in non-responders compared with responders before and after 1 month of treatment. The difference increased to 2.3-fold after 3 months of treatment (P < 0.05, Fig. 1h and Table 3).
Since GM-CSF, TNFα and IL-1β produced by T cells were higher in responder patients compared with non-responder patients, we carried out a cluster analysis to assess their predictive value of responsiveness to anti-TNFα pre-treatment. A two-dimensional clustering was carried out with Pearson’s correlation and complete linkage analysis (Fig. 2a). Two clusters emerged, one containing patients with high levels of GM-CSF, IL1β and TNFα (connected with a blue bracket) and the second containing patients with low levels of the cytokines (connected with a red bracket). Eighteen of 19 patients within the first cluster (94.7%) were responders and only one was a non-responder. The second cluster contained 19 non-responders (57.6%) and 14 responders (42.4%) (Fig. 2c). The relative prevalence of responders to non-responders in each cluster was significantly different (Fisher’s exact test, P < 0.001). Inclusion of the other T cell-derived cytokines measured in this study did not improve the clustering and were, therefore, not included in this analysis.
Cluster and receiver operating characteristic (ROC) analyses for predictive value of cytokines in the response to anti-TNFα. a, b Cluster analyses of TNFα, IL-1β and GM-CSF in RA patients pre-treatment with anti-TNFα in relation to responsiveness/non-responsiveness. Cluster analyses of GM-CSF, IL-1β and TNFα levels (a) by in vitro activated T cells from responder (RES) and non-responder (N_R) patients whose identity codes are indicated by numbers and (b) in plasma from the same patients. The cluster analyses were performed using Pearson’s correlation with complete linkage. The two clusters in a were highlighted with a blue (cluster 1) and a red (cluster 2) bracket. c Summary of data from the cluster in a. Significant differences between responders and non-responders were calculated using Fisher’s exact test. d ROC curve analyses of the response to treatment with anti-TNFα. The increasing area under the ROC curve (area under the curve, AUC) corresponds to a higher diagnostic test yield. e Summary of sensitivity, specificity and odds ratio (OR) for GM-CSF levels by T cells and in plasma before treatment in predicting the response to treatment with anti-TNFα. PPV positive predictive value, NPV negative predictive value. Data are presented with 95% CI
High Plasma GM-CSF Levels Confirms Association with Responsiveness to Anti-TNFα
To explore the feasibility of developing a strategy for identifying likely responders to anti-TNFα before treatment based on the findings from the current study, we also assessed plasma levels of TNFα, IL-1β, IL-6, GM-CSF and MCP-1 pre-treatment (Figs. 2b and 3a–e). There were no differences between responder and non-responder patients in pre-treatment plasma levels of TNFα, IL-6 or MCP-1. However, high pre-treatment levels of GM-CSF were significantly associated with responsiveness to anti-TNFα (6.4 ± 4.6 pg/mL vs. 2.8 ± 2.0 pg/mL, responders vs. non-responders; P < 0.05; Fig. 3d). To assess the potential application of measuring GM-CSF in plasma and/or culture supernatants of T cells before treatment to predict responsiveness, we carried out a receiver operated characteristics (ROC) analysis by plotting the true positive rate (Sensitivity) as a function of the false positive rate (100-Specificity). The analyses revealed that the level of GM-CSF in culture supernatants of activated T cells gave a predictive value, area under curve (AUC), of 0.70, (0.56–0.85 at 95% confidence interval (CI); P < 0.05), and for plasma an AUC of 0.73 (0.53–0.90% at 95% CI; P = 0.09; Fig. 2d). Calculation of odds ratio (OR) showed that responder patients had an OR of 3.9 to respond to treatment at GM-CSF levels of >2.5 pg/mL produced by 200 cultured T cells and an OR of 11.7 at plasma levels of >3.5 pg/mL compared with non-responders (Fig. 2e). The predictive value of GM-CSF in culture supernatants of T cells had a specificity of 73.3% and a sensitivity of 58.3% (40.8–74.5 at 95% CI). For plasma GM-CSF, specificity was 71.4% (29.0–96.3% at 95% CI) and sensitivity 82.4% (56.6–96.2% at 95% CI). The positive predictive values, at cut-offs as stated above, for GM-CSF in culture supernatants of T cells was 84.0% (63.9 to 95.5% at 95% CI) and for plasma 87.5% (61.6 to 98.5% at 95% CI).
Levels of cytokines in plasma pre-treatment of RA patients with anti-TNFα. Plasma levels of cytokines were determined immediately before treatment with anti-TNFα using MSD multiplex kits. Levels of cytokines shown are for: a TNFα; b IL-1β; c IL-6; d GM-CSF and e MCP-1. Values are presented as the mean ± SEM in the plasma of 40 responder and 13 non-responder patients on whom plasma samples were available. Statistical analyses were with the two-tailed Mann-Whitney U test. Single asterisk indicates P < 0.05; double asterisk indicates P < 0.01
The Phenotype and Activation Status of GM-CSF+ T Cells in RA Patients
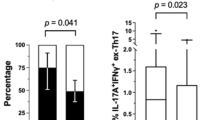
There is evidence that GM-CSF can be produced by CD161+IFNγ+ ex-Th17 cells in the joint of some RA patients and by peripheral blood Th17 cells in patients with multiple sclerosis [33, 34]. Other studies, however, have shown that GM-CSF is produced by T cells other than Th17 cells [35, 36]. To assess the existence of links between GM-CSF-producing T cells and Th17 cells in our cohort of patients in relation to responsiveness to anti-TNFα, T cells were enriched, stimulated with PMA/ionomycin for 16 h, stained for membrane proteins and intracellular cytokines and analysed by FACS. We used CCR6 expression, a membrane protein expressed on the majority of IL-17+ T cells, to identify Th17 cells in the patients (Fig. 4a). To determine whether GM-CSF+ T cells overlapped with Th17 cells, we gated on CCR6+IL-17+ T cells within the enriched/stimulated CD3+ T cells. The FACS contour plots first confirmed that almost all of the IL-17+ T cells expressed CCR6 (2.2% of all CD4+ T cells; Fig. 4a). The gated CCR6+IL-17+ T cells were then analysed for membrane CD161 and intracellular TNFα, GM-CSF and IFNγ expression. This analysis showed that of the CCR6+IL-17+ T cells, 58.0 ± 12.7% and 57.0 ± 16.1% expressed CD161, 19.6 ± 11.7% and 41.9 ± 33.7% expressed IFNγ, 79.3 ± 9.6% and 85.3 ± 2.5% expressed TNFα but only 12.3 ± 4.8% and 13.6 ± 6.6% expressed GM-CSF in responder and non-responder patients, respectively. In contrast, analysis of GM-CSF+ T cells showed that almost all co-expressed TNFα+ (example of FACS contour plots in Fig. 4b). The GM-CSF+TNFα+ T cells (4.9% of all CD3+ T cells) were then gated and analysed for membrane CD161 and intracellular IFNγ and IL-17 expression (Fig. 4b). This analysis showed that of the GM-CSF+TNFα+ T cells 12.1 ± 7.0% and 20.1 ± 4.9% expressed CD161, 32.2 ± 12.2% and 45.4 ± 14.9% expressed IFNγ and 3.7 ± 1.0% and 5.0 ± 1.4% expressed IL-17 in responders and non-responders, respectively (Fig. 4b). Further analysis of GM-CSF+ T cells showed that they were mostly of the CD45RO+ effector/memory phenotype (Fig. 4c). As revealed in Fig. 4b, a relatively small proportion expressed low levels of CD161 but none expressed IL-10 (not shown) indicating that they were unlikely to be regulatory T cells (Tregs) [37, 38]. Thus, although a few Th17 cells produced GM-CSF, the majority did not (P < 0.0001). Likewise, only few cells within the GM-CSF+ TNFα+ T cell population produced IL-17 (p < 0.0001). Furthermore, when the level of IL-17 and GM-CSF from in vitro anti-CD3/CD28-activated T cell culture supernatants was compared in a subgroups of the patients, none of the patients simultaneously produced high level of both cytokines (Fig. 4d).
GM-CSF+ and IL-17+ T cells segregate into two different subsets. Enriched T cells were stimulated with PMA/Ionomycin for 16 h and stained for membrane proteins and intracellular cytokines as indicated. a Contour plot showing the percentage of CCR6+ and CCR6+IL-17+ in blood CD4+ cells and b TNFα+ and TNF+GM-CSF+ T cells in the blood of RA patients. CCR6+IL-17+ in a were gated and the frequency of T cells expressing intracellular GM-CSF, TNFα and IFNγ+ and membrane CD161 determined by FACS. b PMA/Ionomycin-stimulated T cells were stained for intracellular TNFα and GM-CSF and the frequency in responder and non-responder patients determined. The frequency of TNFα+GM-CSF+ T cells that co-expressed INFγ and IL-17 membrane CD161 was determined by multicolour FACS. The data represent the mean ± SEM values from seven responder and three non-responder patients. c Contour plots showing characteristics of the RA patients GM-CSF+ T cells (expression of CD45RA, CD45RO and CD161) pre-treatment. Percentages of differentially stained cell subsets are given inside the quadrants. d Linear regression showing the correlation between the level of IL-17 and GM-CSF produced by patients’ T cells activated with anti-CD3/anti-CD28 mAbs
Discussion
This study provides evidence that the response/lack of response of RA patients to anti-TNFα therapy is associated with differences in the frequency of T cell subsets in patients. Thus, the results show that responder patients tend to have a high frequency of T cells that produce GM-CSF in contrast to non-responders that have a high frequency of IL-17-producing T cells at the start of treatment with anti-TNFα. Furthermore, clustering analysis of the pro-inflammatory cytokines TNFα, GM-CSF and IL-1β suggests that the disease in responder and non-responder patients is likely to be driven/sustained through different inflammatory pathways. The study also suggests that T cells could be the major producers of TNFα and GM-CSF in RA patients. Consistent with the association between high frequency of GM-CSF+ T cells that produce high levels, the cytokine when activated in vitro and responsiveness to TNFα inhibitors is that high plasma level of GM-CSF is also detected in the patients. High level of IL-17 prior to treatment, in contrast, has an inverse relationship with responsiveness to anti-TNFα [11, 12].
About 30% of RA patients prescribed anti-TNFα do not respond adequately and a further 50% of responders relapse within 12 months of treatment [39]. Previous studies from our laboratory have revealed an association between the lack of response to anti-TNFα and high levels of IL-17-producing T cells pre-treatment [12]. These studies also showed an increase in the frequency of Th17 cells after treatment. This latter observation could be related to the fact that TNFα also has immunomodulatory functions [40]. For example, TNFα has been shown to modulate diabetes, EAE, lupus and arthritis in animal models, possibly, by inhibiting Th17 cells [41,42,43,44]. Importantly, findings from the current study show that T cells could be a major source of TNFα in vivo. This finding is consistent with studies showing that both macrophages and T cells in the synovial lining tissue produce TNFα [30, 32]. Since T cells and monocytes express TNFα receptors, the cytokine could act directly on these cells and modulate their differentiation and functions [14, 44]. Indeed, blockade of TNFα in arthritic mice led to the expansion of collagen-specific Th1 and Th17 cells and our previous studies showed that this effect is likely to be due to increased IL-12/IL-23 p40 production by monocytes and antigen-presenting cells [12, 44].
There is clearly an unmet need to identify biomarkers for evidence-based prescription of biological therapies in all RA patients. Identifying such biomarkers has been the subject of much research by many investigators. Several potential biomarkers have been reported (Table 1), but the application of such biomarkers in larger populations beyond the cohorts studied is unknown. For example, anti-cyclic citrullinated peptide antibodies and rheumatoid factors, autoantibodies traditionally associated with RA, were studied but ultimately shown to be of limited value [18, 45]. We propose that a combination of reported biomarkers, for example GM-CSF and the inflammatory protein MRP8/14 or certain micro-RNAs, could predict responsiveness to anti-TNFα in most RA patient cohorts more reproducibly [22, 26].
GM-CSF is an important cytokine for the maturation of macrophages and CD1+ pro-inflammatory dendritic cells [36, 46] as well as in myelopoiesis [47]. Interestingly, treatment with GM-CSF has been shown to precipitate arthritis in some cancer patients [48]. Nevertheless, although GM-CSF has well-characterised pro-inflammatory properties, it is not established if GM-CSF+ T cells are always pathogenic [49]. Thus, the cytokine is associated with a better prognosis in some animal models of auto-immune diseases. For example, GM-CSF has been shown to enhance the ability of dendritic cells to promote Treg cell activity and to ameliorate experimental thyroiditis and diabetes in mice [50, 51]. Although some studies have shown that GM-CSF could be co-produced by IL-17-producing T cells [33, 34], our data and other recent studies indicate that none, or only low levels, of GM-CSF are produced by Th17 cells [34, 35, 52].
Our findings also provide support for the proposition that arthritis could be driven/sustained by different inflammatory pathways in different patients resulting in a heterogeneous response to TNFα inhibitors. The lack of responsiveness to anti-TNFα in patients with low plasma levels of GM-CSF/low-frequency GM-CSF+ T cells indicates that disease processes in these patients are unlikely to be dominated by a pathway driven by TNFα/GM-CSF. However, an alternative explanation could be that anti-TNFα ameliorates arthritis in responder patients by binding to autoreactive effector T cells that express membrane TNFα and thereby ameliorate their pro-inflammatory effects. In non-responder patients, in contrast, it is likely that IL-1β and IL-23 produced by monocytes, when TNFα is blocked, drive Th17 cell differentiation and/or survival and promote chronic inflammation through a different pathway [12].
The current study has also revealed that monocytes from non-responder patients produce higher MCP-1 levels compared with responder patients. A significant difference was seen after 3 months of treatment, which may be accounted for by the possibility that monocytes in anti-TNFα non-responders are resistant to apoptosis [53]. However, further studies of monocytes in responder and non-responder RA patients could elucidate mechanisms that drive inflammation in patient subsets. Such studies may also reveal if there is a relationship between high IL-17 levels in non-responder patients and high-level MCP-1 production by monocytes [54]. All in all, our study is consistent with other studies published in the current issue to indicate that assessment of immune cell subsets could identify biomarkers that help in designing evidence-based strategies for prescribing the most effective biological therapies in autoimmune diseases [55, 56].
Conclusions
This study shows that different T cell subsets are likely involved in driving arthritis in different RA patients and that depending on which T cell subset predominates, determines which patient responds to anti-TNFα. In addition to its potential clinical relevance, the study provides insights into distinct inflammatory pathways that could drive the disease and help in future patient stratification for treatment. Further in-depth studies of GM-CSF+TNFα+ T cells and IL-17-producing T cells, monocyte subsets, their proteome, transcriptome and genotype could provide invaluable new markers for targeted therapies in RA.
References
McInnes IB, Schett G (2011) The pathogenesis of rheumatoid arthritis. N Engl J Med 365(23):2205–2219
Suzuki A, Kochi Y, Okada Y, Yamamoto K (2011) Insight from genome-wide association studies in rheumatoid arthritis and multiple sclerosis. FEBS Lett 585(23):3627–3632
Elliott MJ, Maini RN, Feldmann M, Long-Fox A, Charles P, Katsikis P, Brennan FM, Walker J, Bijl H, Ghrayeb J et al (1993) Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor alpha. Arthritis Rheum 36(12):1681–1690
Emery P, Breedveld FC, Hall S, Durez P, Chang DJ, Robertson D, Singh A, Pedersen RD, Koenig AS, Freundlich B (2008) Comparison of methotrexate monotherapy with a combination of methotrexate and etanercept in active, early, moderate to severe rheumatoid arthritis (COMET): a randomised, double-blind, parallel treatment trial. Lancet 372(9636):375–382
Klarenbeek NB, Allaart CF, Kerstens PJ, Huizinga TW, Dijkmans BA (2009) The BeSt story: on strategy trials in rheumatoid arthritis. Curr Opin Rheumatol 21(3):291–298
Nie H, Zheng Y, Li R, Guo TB, He D, Fang L, Liu X, Xiao L, Chen X, Wan B et al (2013) Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat Med 19(3):322–328
McGovern JL, Nguyen DX, Notley CA, Mauri C, Isenberg DA, Ehrenstein MR (2012) Th17 cells are restrained by Treg cells via the inhibition of interleukin-6 in patients with rheumatoid arthritis responding to anti-tumor necrosis factor antibody therapy. Arthritis Rheum 64(10):3129–3138
Evans HG, Roostalu U, Walter GJ, Gullick NJ, Frederiksen KS, Roberts CA, Sumner J, Baeten DL, Gerwien JG, Cope AP et al (2014) TNF-alpha blockade induces IL-10 expression in human CD4+ T cells. Nat Commun 5:3199
Cope AP, Liblau RS, Yang XD, Congia M, Laudanna C, Schreiber RD, Probert L, Kollias G, McDevitt HO (1997) Chronic tumor necrosis factor alters T cell responses by attenuating T cell receptor signaling. J Exp Med 185(9):1573–1584
Cope AP (2008) T cells in rheumatoid arthritis. Arthritis Res Ther Ther 10(Suppl 1):S1
Chen DY, Chen YM, Chen HH, Hsieh CW, Lin CC, Lan JL (2011) Increasing levels of circulating Th17 cells and interleukin-17 in rheumatoid arthritis patients with an inadequate response to anti-TNF-α therapy. Arthritis Res Ther 13(4):R126
Alzabin S, Abraham SM, Taher TE, Palfreeman A, Hull D, McNamee K, Jawad A, Pathan E, Kinderlerer A, Taylor PC et al (2012) Incomplete response of inflammatory arthritis to TNFalpha blockade is associated with the Th17 pathway. Ann Rheum Dis 71(10):1741–1748
Kawashima M, Miossec P (2005) Effect of treatment of rheumatoid arthritis with infliximab on IFN gamma, IL4, T-bet, and GATA-3 expression: link with improvement of systemic inflammation and disease activity. Ann Rheum Dis 64(3):415–418
Bystrom J, Clanchy FI, Taher TE, Mangat P, Jawad AS, Williams RO, Mageed RA (2016) TNFα in the regulation of Treg and Th17 cells in rheumatoid arthritis and other autoimmune inflammatory diseases. Cytokine pii S1043-4666(16):30490–30492
Fabre S, Dupuy AM, Dossat N, Guisset C, Cohen JD, Cristol JP, Daures JP, Jorgensen C (2008) Protein biochip array technology for cytokine profiling predicts etanercept responsiveness in rheumatoid arthritis. Clin Exp Immunol 153(2):188–195
Kayakabe K, Kuroiwa T, Sakurai N, Ikeuchi H, Kadiombo AT, Sakairi T, Kaneko Y, Maeshima A, Hiromura K, Nojima Y (2012) Interleukin-1β measurement in stimulated whole blood cultures is useful to predict response to anti-TNF therapies in rheumatoid arthritis. Rheumatology 51(9):1639–1643
Wampler Muskardin T, Vashisht P, Dorschner JM, Jensen MA, Chrabot BS, Kern M, Curtis JR, Danila MI, Cofield SS, Shadick N et al (2016) Increased pretreatment serum IFN-β/α ratio predicts non-response to tumour necrosis factor α inhibition in rheumatoid arthritis. Ann Rheum Dis 75(10):1757–1762
Hueber W, Tomooka BH, Batliwalla F, Li W, Monach PA, Tibshirani RJ, Van Vollenhoven RF, Lampa J, Saito K, Tanaka Y et al (2009) Blood autoantibody and cytokine profiles predict response to anti-tumor necrosis factor therapy in rheumatoid arthritis. Arthritis Res Ther 11(3):R76
Greisen SR, Schelde KK, Rasmussen TK, Kragstrup TW, Stengaard-Pedersen K, Hetland ML, Hørslev-Petersen K, Junker P, Østergaard M, Deleuran B et al (2014) CXCL13 predicts disease activity in early rheumatoid arthritis and could be an indicator of the therapeutic ‘window of opportunity’. Arthritis Res Ther 16(5):434
Han BK, Kuzin I, Gaughan JP, Olsen NJ, Bottaro A (2016) Baseline CXCL10 and CXCL13 levels are predictive biomarkers for tumor necrosis factor inhibitor therapy in patients with moderate to severe rheumatoid arthritis: a pilot, prospective study. Arthritis Res Ther 18:93
Dennis G Jr, Holweg CT, Kummerfeld SK, Choy DF, Setiadi AF, Hackney JA, Haverty PM, Gilbert H, Lin WY, Diehl L et al (2014) Synovial phenotypes in rheumatoid arthritis correlate with response to biologic therapeutics. Arthritis Res Ther 16(2):R90
Choi IY, Gerlag DM, Herenius MJ, Thurlings RM, Wijbrandts CA, Foell D, Vogl T, Roth J, Tak PP, Holzinger D (2015) MRP8/14 serum levels as a strong predictor of response to biological treatments in patients with rheumatoid arthritis. Ann Rheum Dis 74(3):499–505
Lequerré T, Gauthier-Jauneau AC, Bansard C, Derambure C, Hiron M, Vittecoq O, Daveau M, Mejjad O, Daragon A, Tron F et al (2006) Gene profiling in white blood cells predicts infliximab responsiveness in rheumatoid arthritis. Arthritis Res Ther 8(4):R105
Julià A, Erra A, Palacio C, Tomas C, Sans X, Barceló P, Marsal S (2009) An eight-gene blood expression profile predicts the response to infliximab in rheumatoid arthritis. PLoS One 4(10):e7556
Folkersen L, Brynedal B, Diaz-Gallo LM, Ramsköld D, Shchetynsky K, Westerlind H, Sundström Y, Schepis D, Hensvold A, Vivar N et al (2016) Integration of known DNA, RNA and protein biomarkers provides prediction of anti-TNF response in rheumatoid arthritis: results from the COMBINE study. Mol Med 22(8):322–328
Castro-Villegas C, Pérez-Sánchez C, Escudero A, Filipescu I, Verdu M, Ruiz-Limón P, Aguirre MA, Jiménez-Gomez Y, Font P, Rodriguez-Ariza A et al (2015) Circulating miRNAs as potential biomarkers of therapy effectiveness in rheumatoid arthritis patients treated with anti-TNFalpha. Arthritis Res Ther 17:49
Krintel SB, Dehlendorff C, Hetland ML, Hørslev-Petersen K, Andersen KK, Junker P, Pødenphant J, Ellingsen T, Ahlquist P, Lindegaard HM et al (2016) Prediction of treatment response to adalimumab: a double-blind placebo-controlled study of circulating microRNA in patients with early rheumatoid arthritis. Pharmacogenomics J 16(2):141–146
Fransen J, van Riel PL (2005) The disease activity score and the EULAR response criteria. Clin Exp Rheumatol 23(5 Suppl 39):S93–S99
Chara L, Sánchez-Atrio A, Pérez A, Cuende E, Albarrán F, Turrión A, Chevarria J, Sánchez MA, Monserrat J, de la Hera A et al (2012) Monocyte populations as markers of response to adalimumab plus MTX in rheumatoid arthritis. Arthritis Res Ther 14(4):R175
Church LD, Filer AD, Hidalgo E, Howlett KA, Thomas AM, Rapecki S, Scheel-Toellner D, Buckley CD, Raza K (2010) Rheumatoid synovial fluid interleukin-17-producing CD4 T cells have abundant tumor necrosis factor-alpha co-expression, but little interleukin-22 and interleukin-23R expression. Arthritis Res Ther 12(5):R184
van der Bruggen T, Nijenhuis S, van Raaij E, Verhoef J, van Asbeck BS (1999) Lipopolysaccharide-induced tumor necrosis factor alpha production by human monocytes involves the raf-1/MEK1-MEK2/ERK1-ERK2 pathway. Infect Immun 67(8):3824–3829
Chu CQ, Field M, Feldmann M, Maini RN (1991) Localization of tumor necrosis factor alpha in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum 34(9):1125–1132
Kara EE, McKenzie DR, Bastow CR, Gregor CE, Fenix KA, Ogunniyi AD, Paton JC, Mack M, Pombal DR, Seillet C et al (2015) CCR2 defines in vivo development and homing of IL-23-driven GM-CSF-producing Th17 cells. Nat Commun 6:8644
Piper C, Pesenacker AM, Bending D, Thirugnanabalan B, Varsani H, Wedderburn LR, Nistala K (2014) T cell expression of granulocyte-macrophage colony-stimulating factor in juvenile arthritis is contingent upon Th17 plasticity. Arthritis Rheum 66(7):1955–1960
Noster R, Riedel R, Mashreghi MF, Radbruch H, Harms L, Haftmann C, Chang HD, Radbruch A, Zielinski CE (2014) IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Sci Transl Med 6(241):241ra80
Reynolds G, Gibbon JR, Pratt AG, Wood MJ, Coady D, Raftery G, Lorenzi AR, Gray A, Filer A, Buckley CD et al (2016) Synovial CD4+ T-cell-derived GM-CSF supports the differentiation of an inflammatory dendritic cell population in rheumatoid arthritis. Ann Rheum Dis 75(5):899–907
Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F (1999) An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med 190(7):995–1004
Maggi L, Santarlasci V, Capone M, Peired A, Frosali F, Crome SQ, Querci V, Fambrini M, Liotta F, Levings MK et al (2010) CD161 is a marker of all human IL-17-producing T-cell subsets and is induced by RORC. Eur J Immunol 40(8):2174–2181
Buch MH, Bingham SJ, Bryer D, Emery P (2007) Long-term infliximab treatment in rheumatoid arthritis: subsequent outcome of initial responders. Rheumatology 46(7):1153–1156
Jacob CO, McDevitt HO (1988) Tumour necrosis factor-alpha in murine autoimmune ‘lupus’ nephritis. Nature 331(6154):356–358
Satoh J, Seino H, Abo T, Tanaka S, Shintani S, Ohta S, Tamura K, Sawai T, Nobunaga T, Oteki T et al (1989) Recombinant human tumor necrosis factor alpha suppresses autoimmune diabetes in nonobese diabetic mice. J Clin Invest 84(4):1345–1348
Liu J, Marino MW, Wong G, Grail D, Dunn A, Bettadapura J, Slavin AJ, Old L, Bernard CC (1998) TNF is a potent anti-inflammatory cytokine in autoimmune-mediated demyelination. Nat Med 4(1):78–83
Jacob N, Yang H, Pricop L, Liu Y, Gao X, Zheng SG, Wang J, Gao HX, Putterman C, Koss MN et al (2009) Accelerated pathological and clinical nephritis in systemic lupus erythematosus-prone New Zealand mixed 2328 mice doubly deficient in TNF receptor 1 and TNF receptor 2 via a Th17-associated pathway. J Immunol 182(4):2532–2541
Notley CA, Inglis JJ, Alzabin S, McCann FE, McNamee KE, Williams RO (2008) Blockade of tumor necrosis factor in collagen-induced arthritis reveals a novel immunoregulatory pathway for Th1 and Th17 cells. J Exp Med 205(11):2491–2497
Potter C, Hyrich KL, Tracey A, Lunt M, Plant D, Symmons DP, Thomson W, Worthington J, Emery P, Morgan AW et al (2009) Association of rheumatoid factor and anti-cyclic citrullinated peptide positivity, but not carriage of shared epitope or PTPN22 susceptibility variants, with anti-tumour necrosis factor response in rheumatoid arthritis. Ann Rheum Dis 68(1):69–74
Cornish AL, Campbell IK, McKenzie BS, Chatfield S, Wicks IP (2009) G-CSF and GM-CSF as therapeutic targets in rheumatoid arthritis. Nat Rev Rheumatol 5(10):554–559
Hamilton JA (2008) Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol 8(7):533–544
de Vries EG, Willemse PH, Biesma B, Stern AC, Limburg PC, Vellenga E (1991) Flare-up of rheumatoid arthritis during GM-CSF treatment after chemotherapy. Lancet 338(8765):517–518
Herndler-Brandstetter D, Flavell RA (2014) Producing GM-CSF: a unique T helper subset? Cell Res 24(12):1379–1380
Ganesh BB, Cheatem DM, Sheng JR, Vasu C, Prabhakar BS (2009) GM-CSF-induced CD11c+CD8a—dendritic cells facilitate Foxp3+ and IL-10+ regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol 21(3):269–282
Gaudreau S, Guindi C, Menard M, Besin G, Dupuis G, Amrani A (2007) Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol 179(6):3638–3647
Sheng W, Yang F, Zhou Y, Yang H, Low PY, Kemeny DM, Tan P, Moh A, Kaplan MH, Zhang Y et al (2014) STAT5 programs a distinct subset of GM-CSF-producing T helper cells that is essential for autoimmune neuroinflammation. Cell Res 24(12):1387–1402
Meusch U, Klingner M, Baerwald C, Rossol M, Wagner U (2013) Deficient spontaneous in vitro apoptosis and increased tmTNF reverse signaling-induced apoptosis of monocytes predict suboptimal therapeutic response of rheumatoid arthritis to TNF inhibition. Arthritis Res Ther 15(6):R219
Shahrara S, Pickens SR, Mandelin AM 2nd, Karpus WJ, Huang Q, Kolls JK, Pope RM (2010) IL-17-mediated monocyte migration occurs partially through CC chemokine ligand 2/monocyte chemoattractant protein-1 induction. J Immunol 184(8):4479–4487
Gazeau P, Alegria GC, Devauchelle-Pensec V, Lemerle J, Bendaoud B, Saraux A, Cornec D, Renaudineau Y (2017) Switched memory B cells influence response to abatacept in rheumatoid arthritis. Clinic Rev Allerg Immunol (In press).
Schreiber K, Nocturne G, Cornec D, Daïen CI (2017) Lymphocytes as biomarkers of therapeutic response in rheumatic autoimmune diseases, a realistic goal? Clinic Rev Allerg Immunol (In press).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This study was supported by the Investigator Initiated Research Grant No. WS872934 from Pfizer to Professor Rizgar A. Mageed.
Ethical Approval
The study summarised in this article was approved by the Ethical Committee of the City-East of London and conducted in compliance with the Declaration of Helsinki 2013. Informed consent was obtained from all individual participants included in the study.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bystrom, J., Clanchy, F.I., Taher, T.E. et al. Response to Treatment with TNFα Inhibitors in Rheumatoid Arthritis Is Associated with High Levels of GM-CSF and GM-CSF+ T Lymphocytes. Clinic Rev Allerg Immunol 53, 265–276 (2017). https://doi.org/10.1007/s12016-017-8610-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-017-8610-y