Abstract
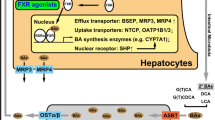
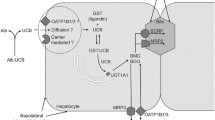
Jaundice results from the systemic accumulation of bilirubin, the final product of the catabolism of haem. Inherited liver disorders of bilirubin metabolism and transport can result in reduced hepatic uptake, conjugation or biliary secretion of bilirubin. In patients with Rotor syndrome, bilirubin (re)uptake is impaired due to the deficiency of two basolateral/sinusoidal hepatocellular membrane proteins, organic anion-transporting polypeptide 1B1 (OATP1B1) and OATP1B3. Dubin-Johnson syndrome is caused by a defect in the ATP-dependent canalicular transporter, multidrug resistance-associated protein 2 (MRP2), which mediates the export of conjugated bilirubin into bile. Both disorders are benign and not progressive and are characterised by elevated serum levels of mainly conjugated bilirubin. Uridine diphospho-glucuronosyl transferase 1A1 (UGT1A1) is responsible for the glucuronidation of bilirubin; deficiency of this enzyme results in unconjugated hyperbilirubinaemia. Gilbert syndrome is the mild and benign form of inherited unconjugated hyperbilirubinaemia and is mostly caused by reduced promoter activity of the UGT1A1 gene. Crigler-Najjar syndrome is the severe inherited form of unconjugated hyperbilirubinaemia due to mutations in the UGT1A1 gene, which can cause kernicterus early in life and can be even lethal when left untreated. Due to major disadvantages of the current standard treatments for Crigler-Najjar syndrome, phototherapy and liver transplantation, new effective therapeutic strategies are under development. Here, we review the clinical features, pathophysiology and genetic background of these inherited disorders of bilirubin metabolism and transport. We also discuss the upcoming treatment option of viral gene therapy for genetic disorders such as Crigler-Najjar syndrome and the possible immunological consequences of this therapy.
Similar content being viewed by others
Abbreviations
- aAbs:
-
Autoantibodies
- AAV:
-
Adeno-associated virus
- AIH:
-
Autoimmune hepatitis
- CN:
-
Crigler-Najjar syndrome
- EPO:
-
Erythropoietin
- GS:
-
Gilbert syndrome
- HSC:
-
Haematopoietic stem cells
- LKM3:
-
Liver-kidney-microsomal antibodies 3
- MRP2:
-
Multidrug resistance-associated protein 2
- OLT:
-
Orthotopic liver transplantation
- RS:
-
Rotor syndrome
- UCB:
-
Unconjugated bilirubin
- UGT1A1:
-
Uridine diphospho-glucuronosyl transferase 1A1
References
Bissell DM (1975) Formation and elimination of bilirubin. Gastroenterology 69:519–538
Berk PD, Howe RB, Bloomer JR, Berlin NI (1969) Studies of bilirubin kinetics in normal adults. J Clin Invest 48:2176–2190
Tenhunen R, Marver HS, Schmid R (1968) The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A 61:748–755
Tenhunen R, Marver HS, Schmid R (1970) The enzymatic catabolism of hemoglobin: stimulation of microsomal heme oxygenase by hemin. J Lab Clin Med 75:410–421
Tenhunen R, Ross ME, Marver HS, Schmid R (1970) Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry 9:298–303, 20-1
Sorrentino D, Berk PD (1988) Mechanistic aspects of hepatic bilirubin uptake. Semin Liver Dis 8:119–136
Tiribelli C, Ostrow JD (1993) New concepts in bilirubin chemistry, transport and metabolism: report of the Second International Bilirubin Workshop, April 9–11, 1992, Trieste, Italy. Hepatology 17:715–736
Schmid R, McDonagh AF (1975) The enzymatic formation of bilirubin. Ann N Y Acad Sci 244:533–552
Dubin IN, Johnson FB (1954) Chronic idiopathic jaundice with unidentified pigment in liver cells; a new clinicopathologic entity with a report of 12 cases. Medicine (Baltimore) 33:155–197
Sprinz H, Nelson RS (1954) Persistent non-hemolytic hyperbilirubinemia associated with lipochrome-like pigment in liver cells: report of four cases. Ann Intern Med 41:952–962
Swartz HM, Sarna T, Varma RR (1979) On the natural and excretion of the hepatic pigment in the Dubin-Johnson syndrome. Gastroenterology 76:958–964
Koskelo P, Toivonen I, Adlercreutz H (1967) Urinary coproporphyrin isomer distribution in the Dubin-Johnson syndrome. Clin Chem 13:1006–1009
Kondo T, Kuchiba K, Shimizu Y (1976) Coproporphyrin isomers in Dubin-Johnson syndrome. Gastroenterology 70:1117–1120
Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RP (1996) Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science 271:1126–1128
Paulusma CC, Kool M, Bosma PJ, Scheffer GL, Ter BF, Scheper RJ, Tytgat GN, Borst P, Baas F, Oude Elferink RP (1997) A mutation in the human canalicular multispecific organic anion transporter gene causes the Dubin-Johnson syndrome. Hepatology 25:1539–1542
Keppler D, Konig J, Buchler M (1997) The canalicular multidrug resistance protein, cMRP/MRP2, a novel conjugate export pump expressed in the apical membrane of hepatocytes. Adv Enzyme Regul 37:321–333
Miller DS, Nobmann SN, Gutmann H, Toeroek M, Drewe J, Fricker G (2000) Xenobiotic transport across isolated brain microvessels studied by confocal microscopy. Mol Pharmacol 58:1357–1367
Borst P, Zelcer N, van de Wetering K (2006) MRP2 and 3 in health and disease. Cancer Lett 234:51–61
Jedlitschky G, Hoffmann U, Kroemer HK (2006) Structure and function of the MRP2 (ABCC2) protein and its role in drug disposition. Expert Opin Drug Metab Toxicol 2:351–366
Dietrich CG, Geier A, Oude Elferink RP (2003) ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut 52:1788–1795
Rotor AB, Manahan L, Florentin A (1948) Familial nonhemolytic jaundice with direct van den Bergh reaction. Acta Med Phil 5:37–49
Shimizu Y, Naruto H, Ida S, Kohakura M (1981) Urinary coproporphyrin isomers in Rotor’s syndrome: a study in eight families. Hepatology 1:173–178
Wolpert E, Pascasio FM, Wolkoff AW, Arias IM (1977) Abnormal sulfobromophthalein metabolism in Rotor’s syndrome and obligate heterozygotes. N Engl J Med 296:1099–1101
van de Steeg E, Stranecky V, Hartmannova H, Noskova L, Hrebicek M, Wagenaar E, van Esch A, de Waart DR, Oude Elferink RP, Kenworthy KE, Sticova E, Al-Edreesi M, Knisely AS, Kmoch S, Jirsa M, Schinkel AH (2012) Complete OATP1B1 and OATP1B3 deficiency causes human Rotor syndrome by interrupting conjugated bilirubin reuptake into the liver. J Clin Invest 122:519–528
Hagenbuch B, Gui C (2008) Xenobiotic transporters of the human organic anion transporting polypeptides (OATP) family. Xenobiotica 38:778–801
Konig J, Cui Y, Nies AT, Keppler D (2000) Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem 275:23161–23168
Crigler JF Jr, Najjar VA (1952) Congenital familial nonhemolytic jaundice with kernicterus. Pediatrics 10:169–180
Sinaasappel M, Jansen PL (1991) The differential diagnosis of Crigler-Najjar disease, types 1 and 2, by bile pigment analysis. Gastroenterology 100:783–789
Arias IM, Gartner LM, Cohen M, Ezzer JB, Levi AJ (1969) Chronic nonhemolytic unconjugated hyperbilirubinemia with glucuronyl transferase deficiency. Clinical, biochemical, pharmacologic and genetic evidence for heterogeneity. Am J Med 47:395–409
Ritter JK, Crawford JM, Owens IS (1991) Cloning of two human liver bilirubin UDP-glucuronosyltransferase cDNAs with expression in COS-1 cells. J Biol Chem 266:1043–1047
Ritter JK, Chen F, Sheen YY, Tran HM, Kimura S, Yeatman MT, Owens IS (1992) A novel complex locus UGT1 encodes human bilirubin, phenol, and other UDP-glucuronosyltransferase isozymes with identical carboxyl termini. J Biol Chem 267:3257–3261
Gong QH, Cho JW, Huang T, Potter C, Gholami N, Basu NK, Kubota S, Carvalho S, Pennington MW, Owens IS, Popescu NC (2001) Thirteen UDPglucuronosyltransferase genes are encoded at the human UGT1 gene complex locus. Pharmacogenetics 11:357–368
Bosma PJ, Seppen J, Goldhoorn B, Bakker C, Oude Elferink RP, Chowdhury JR, Chowdhury NR, Jansen PL (1994) Bilirubin UDP-glucuronosyltransferase 1 is the only relevant bilirubin glucuronidating isoform in man. J Biol Chem 269:17960–17964
Aono S, Yamada Y, Keino H, Hanada N, Nakagawa T, Sasaoka Y, Yazawa T, Sato H, Koiwai O (1993) Identification of defect in the genes for bilirubin UDP-glucuronosyl-transferase in a patient with Crigler-Najjar syndrome type II. Biochem Biophys Res Commun 197:1239–1244
Ritter JK, Yeatman MT, Ferreira P, Owens IS (1992) Identification of a genetic alteration in the code for bilirubin UDP-glucuronosyltransferase in the UGT1 gene complex of a Crigler-Najjar type I patient. J Clin Invest 90:150–155
Bosma PJ, Chowdhury NR, Goldhoorn BG, Hofker MH, Oude Elferink RP, Jansen PL, Chowdhury JR (1992) Sequence of exons and the flanking regions of human bilirubin-UDP-glucuronosyltransferase gene complex and identification of a genetic mutation in a patient with Crigler-Najjar syndrome, type I. Hepatology 15:941–947
Canu G, Minucci A, Zuppi C, Capoluongo E (2013) Gilbert and Crigler Najjar syndromes: an update of the UDP-glucuronosyltransferase 1A1 (UGT1A1) gene mutation database. Blood Cells Mol Dis 50:273–280
Meulengracht E (1947) A review of chronic intermittent juvenile jaundice. Q J Med 16:83–98
Gilbert ALP (1901) La cholamae simple familiale. Sem Med 21:241
Bosma PJ, Chowdhury JR, Bakker C, Gantla S, de Boer A, Oostra BA, Lindhout D, Tytgat GN, Jansen PL, Oude Elferink RP (1995) The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. N Engl J Med 333:1171–1175
Raijmakers MT, Jansen PL, Steegers EA, Peters WH (2000) Association of human liver bilirubin UDP-glucuronyltransferase activity with a polymorphism in the promoter region of the UGT1A1 gene. J Hepatol 33:348–351
Black M, Billing BH (1968) ‘In vitro’ assay of bilirubin-UDP glucuronyl transferase activity in the liver of patients with Gilbert’s syndrome and a variety of hepatic disorders. Gut 9:728–729
Black M, Billing BH (1969) Hepatic bilirubin UDP-glucuronyl transferase activity in liver disease and Gilbert’s syndrome. N Engl J Med 280:1266–1271
Monaghan G, Ryan M, Seddon R, Hume R, Burchell B (1996) Genetic variation in bilirubin UPD-glucuronosyltransferase gene promoter and Gilbert’s syndrome. Lancet 347:578–581
Hall D, Ybazeta G, Destro-Bisol G, Petzl-Erler ML, Di RA (1999) Variability at the uridine diphosphate glucuronosyltransferase 1A1 promoter in human populations and primates. Pharmacogenetics 9:591–599
Sato H, Adachi Y, Koiwai O (1996) The genetic basis of Gilbert’s syndrome. Lancet 347:557–558
Soeda Y, Yamamoto K, Adachi Y, Hori T, Aono S, Koiwai O, Sato H (1995) Predicted homozygous mis-sense mutation in Gilbert’s syndrome. Lancet 346:1494
Miners JO, Mackenzie PI (1991) Drug glucuronidation in humans. Pharmacol Ther 51:347–369
Ghosal A, Hapangama N, Yuan Y, Achanfuo-Yeboah J, Iannucci R, Chowdhury S, Alton K, Patrick JE, Zbaida S (2004) Identification of human UDP-glucuronosyltransferase enzyme(s) responsible for the glucuronidation of ezetimibe (Zetia). Drug Metab Dispos 32:314–320
Ebner T, Remmel RP, Burchell B (1993) Human bilirubin UDP-glucuronosyltransferase catalyzes the glucuronidation of ethinylestradiol. Mol Pharmacol 43:649–654
McGill MR, Jaeschke H (2013) Metabolism and disposition of acetaminophen: recent advances in relation to hepatotoxicity and diagnosis. Pharm Res 30:2174–2187
Prueksaritanont T, Subramanian R, Fang X, Ma B, Qiu Y, Lin JH, Pearson PG, Baillie TA (2002) Glucuronidation of statins in animals and humans: a novel mechanism of statin lactonization. Drug Metab Dispos 30:505–512
Tukey RH, Strassburg CP, Mackenzie PI (2002) Pharmacogenomics of human UDP-glucuronosyltransferases and irinotecan toxicity. Mol Pharmacol 62:446–450
Innocenti F, Undevia SD, Iyer L, Chen PX, Das S, Kocherginsky M, Karrison T, Janisch L, Ramirez J, Rudin CM, Vokes EE, Ratain MJ (2004) Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 22:1382–1388
Innocenti F, Ratain MJ (2003) Irinotecan treatment in cancer patients with UGT1A1 polymorphisms. Oncology (Williston Park) 17:52–55
Zucker SD, Qin X, Rouster SD, Yu F, Green RM, Keshavan P, Feinberg J, Sherman KE (2001) Mechanism of indinavir-induced hyperbilirubinemia. Proc Natl Acad Sci U S A 98:12671–12676
Lankisch TO, Behrens G, Ehmer U, Mobius U, Rockstroh J, Wehmeier M, Kalthoff S, Freiberg N, Manns MP, Schmidt RE, Strassburg CP (2009) Gilbert’s syndrome and hyperbilirubinemia in protease inhibitor therapy—an extended haplotype of genetic variants increases risk in indinavir treatment. J Hepatol 50:1010–1018
Shapiro SM, Bhutani VK, Johnson L (2006) Hyperbilirubinemia and kernicterus. Clin Perinatol 33:387–410
Watchko JF, Tiribelli C (2013) Bilirubin-induced neurologic damage—mechanisms and management approaches. N Engl J Med 369:2021–2030
Poddar B, Bharti B, Goraya J, Parmar VR (2002) Kernicterus in a child with Crigler-Najjar syndrome type II. Trop Gastroenterol 23:33–34
Cremer RJ, Perryman PW, Richards DH (1958) Influence of light on the hyperbilirubinaemia of infants. Lancet 1:1094–1097
Ennever JF (1990) Blue light, green light, white light, more light: treatment of neonatal jaundice. Clin Perinatol 17:467–481
Zenone EA, Stoll MS, Ostrow JD (1982) The effect of elimination of environmental light on the metabolism of unconjugated bilirubin in the Gunn rat. Dig Dis Sci 27:1117–1120
Stoll MS, Zenone EA, Ostrow JD (1981) Excretion of administered and endogenous photobilirubins in the bile of the jaundice Gunn rat. J Clin Invest 68:134–141
Van Der Veere CN, Sinaasappel M, McDonagh AF, Rosenthal P, Labrune P, Odievre M, Fevery J, Otte JB, McClean P, Burk G, Masakowski V, Sperl W, Mowat AP, Vergani GM, Heller K, Wilson JP, Shepherd R, Jansen PL (1996) Current therapy for Crigler-Najjar syndrome type 1: report of a world registry. Hepatology 24:311–315
Van Der Veere CN, Schoemaker B, Bakker C, Van Der Meer R, Jansen PL, Elferink RP (1996) Influence of dietary calcium phosphate on the disposition of bilirubin in rats with unconjugated hyperbilirubinemia. Hepatology 24:620–626
Stoll MS (1986) Phototherapy of jaundice. In: Ostrow JD (ed) Bile pigments and jaundice. Raven, New York, pp 551–580
Yohannan MD, Terry HJ, Littlewood JM (1983) Long term phototherapy in Crigler-Najjar syndrome. Arch Dis Child 58:460–462
Strauss KA, Robinson DL, Vreman HJ, Puffenberger EG, Hart G, Morton DH (2006) Management of hyperbilirubinemia and prevention of kernicterus in 20 patients with Crigler-Najjar disease. Eur J Pediatr 165:306–319
Muiesan P, Vergani D, Mieli-Vergani G (2007) Liver transplantation in children. J Hepatol 46:340–348
Fox IJ, Chowdhury JR, Kaufman SS, Goertzen TC, Chowdhury NR, Warkentin PI, Dorko K, Sauter BV, Strom SC (1998) Treatment of the Crigler-Najjar syndrome type I with hepatocyte transplantation. N Engl J Med 338:1422–1426
Ambrosino G, Varotto S, Strom SC, Guariso G, Franchin E, Miotto D, Caenazzo L, Basso S, Carraro P, Valente ML, D’Amico D, Zancan L, D’Antiga L (2005) Isolated hepatocyte transplantation for Crigler-Najjar syndrome type 1. Cell Transplant 14:151–157
Lysy PA, Najimi M, Stephenne X, Bourgois A, Smets F, Sokal EM (2008) Liver cell transplantation for Crigler-Najjar syndrome type I: update and perspectives. World J Gastroenterol 14:3464–3470
Kaufmann KB, Buning H, Galy A, Schambach A, Grez M (2013) Gene therapy on the move. EMBO Mol Med 5:1642–1661
Blaese RM, Culver KW, Miller AD, Carter CS, Fleisher T, Clerici M, Shearer G, Chang L, Chiang Y, Tolstoshev P, Greenblatt JJ, Rosenberg SA, Klein H, Berger M, Mullen CA, Ramsey WJ, Muul L, Morgan RA, Anderson WF (1995) T lymphocyte-directed gene therapy for ADA- SCID: initial trial results after 4 years. Science 270:475–480
Gaspar HB, Thrasher AJ (2005) Gene therapy for severe combined immunodeficiencies. Expert Opin Biol Ther 5:1175–1182
Cavazzana-Calvo M, Fischer A, Hacein-Bey-Abina S, Aiuti A (2012) Gene therapy for primary immunodeficiencies: part 1. Curr Opin Immunol 24:580–584
van der Putten H, Quint W, van Raaij J, Maandag ER, Verma IM, Berns A (1981) M-MuLV-induced leukemogenesis: integration and structure of recombinant proviruses in tumors. Cell 24:729–739
Cavazza A, Moiani A, Mavilio F (2013) Mechanisms of retroviral integration and mutagenesis. Hum Gene Ther 24:119–131
Montini E, Cesana D, Schmidt M, Sanvito F, Bartholomae CC, Ranzani M, Benedicenti F, Sergi LS, Ambrosi A, Ponzoni M, Doglioni C, Di Serio C, von Kalle C, Naldini L (2009) The genotoxic potential of retroviral vectors is strongly modulated by vector design and integration site selection in a mouse model of HSC gene therapy. J Clin Invest 119:964–975
Sibbald B (2001) Death but one unintended consequence of gene-therapy trial. CMAJ 164:1612
Peng Z (2005) Current status of gendicine in China: recombinant human Ad-p53 agent for treatment of cancers. Hum Gene Ther 16:1016–1027
Wilson JM (2005) Gendicine: the first commercial gene therapy product. Hum Gene Ther 16:1014–1015
Sheridan C (2011) Gene therapy finds its niche. Nat Biotechnol 29:121–128
Hoggan MD, Blacklow NR, Rowe WP (1966) Studies of small DNA viruses found in various adenovirus preparations: physical, biological, and immunological characteristics. Proc Natl Acad Sci U S A 55:1467–1474
Schnepp BC, Jensen RL, Chen CL, Johnson PR, Clark KR (2005) Characterization of adeno-associated virus genomes isolated from human tissues. J Virol 79:14793–14803
Gaudet D, de Wal J, Tremblay K, Dery S, van Deventer S, Freidig A, Brisson D, Methot J (2010) Review of the clinical development of alipogene tiparvovec gene therapy for lipoprotein lipase deficiency. Atheroscler Suppl 11:55–60
Buning H (2013) Gene therapy enters the pharma market: the short story of a long journey. EMBO Mol Med 5:1–3
Gao G, Vandenberghe LH, Wilson JM (2005) New recombinant serotypes of AAV vectors. Curr Gene Ther 5:285–297
Mingozzi F, High KA (2011) Immune responses to AAV in clinical trials. Curr Gene Ther 11:321–330
Nathwani AC, Tuddenham EG, Rangarajan S, Rosales C, McIntosh J, Linch DC, Chowdary P, Riddell A, Pie AJ, Harrington C, O’Beirne J, Smith K, Pasi J, Glader B, Rustagi P, Ng CY, Kay MA, Zhou J, Spence Y, Morton CL, Allay J, Coleman J, Sleep S, Cunningham JM, Srivastava D, Basner-Tschakarjan E, Mingozzi F, High KA, Gray JT, Reiss UM, Nienhuis AW, Davidoff AM (2011) Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med 365:2357–2365
Miranda PS, Bosma PJ (2009) Towards liver-directed gene therapy for Crigler-Najjar syndrome. Curr Gene Ther 9:72–82
Gunn CK (1944) Hereditary acholuric jaundice in the rat. Can Med Assoc J 50:230–237
Schmid R, Axelrod J, Hammaker L, Swarm RL (1958) Congenital jaundice in rats, due to a defect in glucuronide formation. J Clin Invest 37:1123–1130
Chowdhury NR, Arias IM, Lederstein M, Chowdhury JR (1986) Substrates and products of purified rat liver bilirubin UDP-glucuronosyltransferase. Hepatology 6:123–128
Tada K, Roy-Chowdhury N, Prasad V, Kim BH, Manchikalapudi P, Fox IJ, van Duijvendijk P, Bosma PJ, Roy-Chowdhury J (1998) Long-term amelioration of bilirubin glucuronidation defect in Gunn rats by transplanting genetically modified immortalized autologous hepatocytes. Cell Transplant 7:607–616
Branchereau S, Ferry N, Myara A, Sato H, Kowai O, Trivin F, Houssin D, Danos O, Heard J (1993) Correction of bilirubin glucuronyl transferase in Gunn rats by gene transfer in the liver using retroviral vectors. Chirurgie 119:642–648
van der Wegen P, Louwen R, Imam AM, Buijs-Offerman RM, Sinaasappel M, Grosveld F, Scholte BJ (2006) Successful treatment of UGT1A1 deficiency in a rat model of Crigler-Najjar disease by intravenous administration of a liver-specific lentiviral vector. Mol Ther 13:374–381
Seppen J, Bakker C, de Jong B, Kunne C, van den Oever K, Vandenberghe K, de Waart R, Twisk J, Bosma P (2006) Adeno-associated virus vector serotypes mediate sustained correction of bilirubin UDP glucuronosyltransferase deficiency in rats. Mol Ther 13:1085–1092
Montenegro-Miranda PS, Paneda A, Ten BL, Duijst S, de Waart DR, Aseguinolaza GG, Bosma PJ (2013) Adeno-associated viral vector serotype 5 poorly transduces liver in rat models. PLoS One 8:e82597
Montenegro-Miranda PS, Pichard V, Aubert D, Ten BL, Duijst S, de Waart DR, Ferry N, Bosma PJ (2014) In the rat liver, adenoviral gene transfer efficiency is comparable to AAV. Gene Ther 21:168–174
Philipp T, Durazzo M, Trautwein C, Alex B, Straub P, Lamb JG, Johnson EF, Tukey RH, Manns MP (1994) Recognition of uridine diphosphate glucuronosyl transferases by LKM-3 antibodies in chronic hepatitis D. Lancet 344:578–581
Strassburg CP, Obermayer-Straub P, Alex B, Durazzo M, Rizzetto M, Tukey RH, Manns MP (1996) Autoantibodies against glucuronosyltransferases differ between viral hepatitis and autoimmune hepatitis. Gastroenterology 111:1576–1586
Bachrich T, Thalhammer T, Jager W, Haslmayer P, Alihodzic B, Bakos S, Hitchman E, Senderowicz AM, Penner E (2001) Characterization of autoantibodies against uridine-diphosphate glucuronosyltransferase in patients with inflammatory liver diseases. Hepatology 33:1053–1059
Keitel V, Burdelski M, Vojnisek Z, Schmitt L, Haussinger D, Kubitz R (2009) De novo bile salt transporter antibodies as a possible cause of recurrent graft failure after liver transplantation: a novel mechanism of cholestasis. Hepatology 50:510–517
Astermark J (2006) Basic aspects of inhibitors to factors VIII and IX and the influence of non-genetic risk factors. Haemophilia 12(Suppl 6):8–13
Seppen J, van Til NP, van der Rijt R, Hiralall JK, Kunne C, Elferink RP (2006) Immune response to lentiviral bilirubin UDP-glucuronosyltransferase gene transfer in fetal and neonatal rats. Gene Ther 13:672–677
Takahashi M, Ilan Y, Chowdhury NR, Guida J, Horwitz M, Chowdhury JR (1996) Long term correction of bilirubin-UDP-glucuronosyltransferase deficiency in Gunn rats by administration of a recombinant adenovirus during the neonatal period. J Biol Chem 271:26536–26542
Askari FK, Hitomi Y, Mao M, Wilson JM (1996) Complete correction of hyperbilirubinemia in the Gunn rat model of Crigler-Najjar syndrome type I following transient in vivo adenovirus-mediated expression of human bilirubin UDP-glucuronosyltransferase. Gene Ther 3:381–388
Gao G, Lebherz C, Weiner DJ, Grant R, Calcedo R, McCullough B, Bagg A, Zhang Y, Wilson JM (2004) Erythropoietin gene therapy leads to autoimmune anemia in macaques. Blood 103:3300–3302
Chenuaud P, Larcher T, Rabinowitz JE, Provost N, Cherel Y, Casadevall N, Samulski RJ, Moullier P (2004) Autoimmune anemia in macaques following erythropoietin gene therapy. Blood 103:3303–3304
LoDuca PA, Hoffman BE, Herzog RW (2009) Hepatic gene transfer as a means of tolerance induction to transgene products. Curr Gene Ther 9:104–114
Montenegro-Miranda PS, Ten BL, Kunne C, de Waart DR, Bosma PJ (2011) Mycophenolate mofetil impairs transduction of single-stranded adeno-associated viral vectors. Hum Gene Ther 22:605–612
Brantly ML, Chulay JD, Wang L, Mueller C, Humphries M, Spencer LT, Rouhani F, Conlon TJ, Calcedo R, Betts MR, Spencer C, Byrne BJ, Wilson JM, Flotte TR (2009) Sustained transgene expression despite T lymphocyte responses in a clinical trial of rAAV1-AAT gene therapy. Proc Natl Acad Sci U S A 106:16363–16368
Brantly ML, Spencer LT, Humphries M, Conlon TJ, Spencer CT, Poirier A, Garlington W, Baker D, Song S, Berns KI, Muzyczka N, Snyder RO, Byrne BJ, Flotte TR (2006) Phase I trial of intramuscular injection of a recombinant adeno-associated virus serotype 2 alphal-antitrypsin (AAT) vector in AAT-deficient adults. Hum Gene Ther 17:1177–1186
Flotte TR, Trapnell BC, Humphries M, Carey B, Calcedo R, Rouhani F, Campbell-Thompson M, Yachnis AT, Sandhaus RA, McElvaney NG, Mueller C, Messina LM, Wilson JM, Brantly M, Knop DR, Ye GJ, Chulay JD (2011) Phase 2 clinical trial of a recombinant adeno-associated viral vector expressing alpha1-antitrypsin: interim results. Hum Gene Ther 22:1239–1247
Worgall S, Sondhi D, Hackett NR, Kosofsky B, Kekatpure MV, Neyzi N, Dyke JP, Ballon D, Heier L, Greenwald BM, Christos P, Mazumdar M, Souweidane MM, Kaplitt MG, Crystal RG (2008) Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther 19:463–474
Wagner JA, Reynolds T, Moran ML, Moss RB, Wine JJ, Flotte TR, Gardner P (1998) Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet 351:1702–1703
Wagner JA, Messner AH, Moran ML, Daifuku R, Kouyama K, Desch JK, Manley S, Norbash AM, Conrad CK, Friborg S, Reynolds T, Guggino WB, Moss RB, Carter BJ, Wine JJ, Flotte TR, Gardner P (1999) Safety and biological efficacy of an adeno-associated virus vector-cystic fibrosis transmembrane regulator (AAV-CFTR) in the cystic fibrosis maxillary sinus. Laryngoscope 109:266–274
Wagner JA, Nepomuceno IB, Messner AH, Moran ML, Batson EP, Dimiceli S, Brown BW, Desch JK, Norbash AM, Conrad CK, Guggino WB, Flotte TR, Wine JJ, Carter BJ, Reynolds TC, Moss RB, Gardner P (2002) A phase II, double-blind, randomized, placebo-controlled clinical trial of tgAAVCF using maxillary sinus delivery in patients with cystic fibrosis with antrostomies. Hum Gene Ther 13:1349–1359
Moss RB, Rodman D, Spencer LT, Aitken ML, Zeitlin PL, Waltz D, Milla C, Brody AS, Clancy JP, Ramsey B, Hamblett N, Heald AE (2004) Repeated adeno-associated virus serotype 2 aerosol-mediated cystic fibrosis transmembrane regulator gene transfer to the lungs of patients with cystic fibrosis: a multicenter, double-blind, placebo-controlled trial. Chest 125:509–521
Aitken ML, Moss RB, Waltz DA, Dovey ME, Tonelli MR, McNamara SC, Gibson RL, Ramsey BW, Carter BJ, Reynolds TC (2001) A phase I study of aerosolized administration of tgAAVCF to cystic fibrosis subjects with mild lung disease. Hum Gene Ther 12:1907–1916
Manno CS, Pierce GF, Arruda VR, Glader B, Ragni M, Rasko JJ, Ozelo MC, Hoots K, Blatt P, Konkle B, Dake M, Kaye R, Razavi M, Zajko A, Zehnder J, Rustagi PK, Nakai H, Chew A, Leonard D, Wright JF, Lessard RR, Sommer JM, Tigges M, Sabatino D, Luk A, Jiang H, Mingozzi F, Couto L, Ertl HC, High KA, Kay MA (2006) Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med 12:342–347
Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, Tai SJ, Ragni MV, Thompson A, Ozelo M, Couto LB, Leonard DG, Johnson FA, McClelland A, Scallan C, Skarsgard E, Flake AW, Kay MA, High KA, Glader B (2003) AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood 101:2963–2972
Maguire AM, Simonelli F, Pierce EA, Pugh EN Jr, Mingozzi F, Bennicelli J, Banfi S, Marshall KA, Testa F, Surace EM, Rossi S, Lyubarsky A, Arruda VR, Konkle B, Stone E, Sun J, Jacobs J, Dell’Osso L, Hertle R, Ma JX, Redmond TM, Zhu X, Hauck B, Zelenaia O, Shindler KS, Maguire MG, Wright JF, Volpe NJ, McDonnell JW, Auricchio A, High KA, Bennett J (2008) Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med 358:2240–2248
Testa F, Maguire AM, Rossi S, Pierce EA, Melillo P, Marshall K, Banfi S, Surace EM, Sun J, Acerra C, Wright JF, Wellman J, High KA, Auricchio A, Bennett J, Simonelli F (2013) Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital amaurosis type 2. Ophthalmology 120:1283–1291
Ashtari M, Cyckowski LL, Monroe JF, Marshall KA, Chung DC, Auricchio A, Simonelli F, Leroy BP, Maguire AM, Shindler KS, Bennett J (2011) The human visual cortex responds to gene therapy-mediated recovery of retinal function. J Clin Invest 121:2160–2168
Simonelli F, Maguire AM, Testa F, Pierce EA, Mingozzi F, Bennicelli JL, Rossi S, Marshall K, Banfi S, Surace EM, Sun J, Redmond TM, Zhu X, Shindler KS, Ying GS, Ziviello C, Acerra C, Wright JF, McDonnell JW, High KA, Bennett J, Auricchio A (2010) Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration. Mol Ther 18:643–650
Bainbridge JW, Smith AJ, Barker SS, Robbie S, Henderson R, Balaggan K, Viswanathan A, Holder GE, Stockman A, Tyler N, Petersen-Jones S, Bhattacharya SS, Thrasher AJ, Fitzke FW, Carter BJ, Rubin GS, Moore AT, Ali RR (2008) Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med 358:2231–2239
Gaudet D, Methot J, Dery S, Brisson D, Essiembre C, Tremblay G, Tremblay K, de Wal J, Twisk J, van den Bulk N, Sier-Ferreira V, van Deventer S (2013) Efficacy and long-term safety of alipogene tiparvovec (AAV1-LPLS447X) gene therapy for lipoprotein lipase deficiency: an open-label trial. Gene Ther 20:361–369
Stroes ES, Nierman MC, Meulenberg JJ, Franssen R, Twisk J, Henny CP, Maas MM, Zwinderman AH, Ross C, Aronica E, High KA, Levi MM, Hayden MR, Kastelein JJ, Kuivenhoven JA (2008) Intramuscular administration of AAV1-lipoprotein lipase S447X lowers triglycerides in lipoprotein lipase-deficient patients. Arterioscler Thromb Vasc Biol 28:2303–2304
Smith BK, Collins SW, Conlon TJ, Mah CS, Lawson LA, Martin AD, Fuller DD, Cleaver BD, Clement N, Phillips D, Islam S, Dobjia N, Byrne BJ (2013) Phase I/II trial of adeno-associated virus-mediated alpha-glucosidase gene therapy to the diaphragm for chronic respiratory failure in Pompe disease: initial safety and ventilatory outcomes. Hum Gene Ther 24:630–640
Mendell JR, Campbell K, Rodino-Klapac L, Sahenk Z, Shilling C, Lewis S, Bowles D, Gray S, Li C, Galloway G, Malik V, Coley B, Clark KR, Li J, Xiao X, Samulski J, McPhee SW, Samulski RJ, Walker CM (2010) Dystrophin immunity in Duchenne’s muscular dystrophy. N Engl J Med 363:1429–1437
Bowles DE, McPhee SW, Li C, Gray SJ, Samulski JJ, Camp AS, Li J, Wang B, Monahan PE, Rabinowitz JE, Grieger JC, Govindasamy L, Agbandje-McKenna M, Xiao X, Samulski RJ (2012) Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol Ther 20:443–455
Mendell JR, Rodino-Klapac LR, Rosales XQ, Coley BD, Galloway G, Lewis S, Malik V, Shilling C, Byrne BJ, Conlon T, Campbell KJ, Bremer WG, Taylor LE, Flanigan KM, Gastier-Foster JM, Astbury C, Kota J, Sahenk Z, Walker CM, Clark KR (2010) Sustained alpha-sarcoglycan gene expression after gene transfer in limb-girdle muscular dystrophy, type 2D. Ann Neurol 68:629–638
Mendell JR, Rodino-Klapac LR, Rosales-Quintero X, Kota J, Coley BD, Galloway G, Craenen JM, Lewis S, Malik V, Shilling C, Byrne BJ, Conlon T, Campbell KJ, Bremer WG, Viollet L, Walker CM, Sahenk Z, Clark KR (2009) Limb-girdle muscular dystrophy type 2D gene therapy restores alpha-sarcoglycan and associated proteins. Ann Neurol 66:290–297
Mendell JR, Rodino-Klapac L, Sahenk Z, Malik V, Kaspar BK, Walker CM, Clark KR (2012) Gene therapy for muscular dystrophy: lessons learned and path forward. Neurosci Lett 527:90–99
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
van Dijk, R., Beuers, U. & Bosma, P.J. Gene Replacement Therapy for Genetic Hepatocellular Jaundice. Clinic Rev Allerg Immunol 48, 243–253 (2015). https://doi.org/10.1007/s12016-014-8454-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-014-8454-7