Abstract
Proper proteostasis is indispensable for the long-term maintenance of hematopoietic stem and progenitor cells (HSPCs). The TRiC/CCT (chaperonin-containing TCP-1) complex, a key constituent of cellular machinery facilitating accurate protein folding, has remained enigmatic in its specific function within HSPCs. Here we show that conditional knockout (KO) of Cct5 significantly impairs the maintenance of murine HSPCs. Primary and secondary transplantation experiments unequivocally demonstrate the incapacity of Cct5 KO HSPCs to reconstitute both myeloid and lymphoid lineage cells in recipient mice, highlighting the pivotal role of the TRiC/CCT complex in governing these cellular lineages. Furthermore, leveraging an integrated approach that merges a Protein–Protein Interaction (PPI) database with RNA sequencing (RNA-seq) data of HSPCs, our analysis reveals intricate interactions between Cct5 and vital transcription factors crucial for HSC homeostasis. Notably, Cct5 engages with MYC, PIAS1, TP53, ESR1, HOXA1, and JUN, intricately regulating the transcriptional landscape governing autophagy, myeloid differentiation, and cytoskeleton organization within HSPCs. Our study unveils the profound significance of TRiC/CCT complex-mediated proteostasis in orchestrating the maintenance and functionality of HSPCs.
Graphical Abstract






Similar content being viewed by others
Data Availability (Data Transparency)
Raw data will be provided upon reasonable request.
Code Availability (Software Application or Custom Code)
Not applicable.
References
Pinho, S., & Frenette, P. S. (2019). Haematopoietic stem cell activity and interactions with the niche. Nature Reviews Molecular Cell Biology, 20, 303–320. https://doi.org/10.1038/s41580-019-0103-9
Bunting, K. D., & Qu, C. K. (2014). The hematopoietic stem cell landscape. Methods in Molecular Biology, 1185, 3–6. https://doi.org/10.1007/978-1-4939-1133-2_1
Kasbekar, M., Mitchell, C. A., Proven, M. A., & Passegue, E. (2023). Hematopoietic stem cells through the ages: A lifetime of adaptation to organismal demands. Cell Stem Cell, 30, 1403–1420. https://doi.org/10.1016/j.stem.2023.09.013
Chua, B. A., & Signer, R. A. J. (2020). Hematopoietic stem cell regulation by the proteostasis network. Current Opinion in Hematology, 27, 254–263. https://doi.org/10.1097/MOH.0000000000000591
Chua, B. A., Lennan, C. J., Sunshine, M. J., Dreifke, D., Chawla, A., Bennett, E. J., & Signer, R. A. J. (2023). Hematopoietic stem cells preferentially traffic misfolded proteins to aggresomes and depend on aggrephagy to maintain protein homeostasis. Cell Stem Cell, 30, 460-472 e466. https://doi.org/10.1016/j.stem.2023.02.010
Kelly, J. J., Tranter, D., Pardon, E., Chi, G., Kramer, H., Happonen, L., Knee, K. M., Janz, J. M., Steyaert, J., Bulawa, C., Paavilainen, V. O., Huiskonen, J. T., & Yue, W. W. (2022). Snapshots of actin and tubulin folding inside the TRiC chaperonin. Nature Structural & Molecular Biology, 29, 420–429. https://doi.org/10.1038/s41594-022-00755-1
Yam, A. Y., Xia, Y., Lin, H. T., Burlingame, A., Gerstein, M., & Frydman, J. (2008). Defining the TRiC/CCT interactome links chaperonin function to stabilization of newly made proteins with complex topologies. Nature Structural & Molecular Biology, 15, 1255–1262. https://doi.org/10.1038/nsmb.1515
Gestaut, D., Roh, S. H., Ma, B., Pintilie, G., Joachimiak, L. A., Leitner, A., Walzthoeni, T., Aebersold, R., Chiu, W., & Frydman, J. (2019). The chaperonin TRiC/CCT associates with prefoldin through a conserved electrostatic interface essential for cellular proteostasis. Cell, 177, 751-765 e715. https://doi.org/10.1016/j.cell.2019.03.012
Pavel, M., Imarisio, S., Menzies, F. M., Jimenez-Sanchez, M., Siddiqi, F. H., Wu, X., Renna, M., O’Kane, C. J., Crowther, D. C., & Rubinsztein, D. C. (2016). CCT complex restricts neuropathogenic protein aggregation via autophagy. Nature Communications, 7, 13821. https://doi.org/10.1038/ncomms13821
Hu, Y., & Smyth, G. K. (2009). ELDA: Extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. Journal of Immunological Methods, 347, 70–78. https://doi.org/10.1016/j.jim.2009.06.008
Wilson, A., Murphy, M. J., Oskarsson, T., Kaloulis, K., Bettess, M. D., Oser, G. M., Pasche, A. C., Knabenhans, C., Macdonald, H. R., & Trumpp, A. (2004). c-Myc controls the balance between hematopoietic stem cell self-renewal and differentiation. Genes & Development, 18, 2747–2763. https://doi.org/10.1101/gad.313104
Liu, B., Yee, K. M., Tahk, S., Mackie, R., Hsu, C., & Shuai, K. (2014). PIAS1 SUMO ligase regulates the self-renewal and differentiation of hematopoietic stem cells. EMBO Journal, 33, 101–113. https://doi.org/10.1002/embj.201283326
Liu, Y., Elf, S. E., Miyata, Y., Sashida, G., Liu, Y., Huang, G., Di Giandomenico, S., Lee, J. M., Deblasio, A., Menendez, S., Antipin, J., Reva, B., Koff, A., & Nimer, S. D. (2009). p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell, 4, 37–48. https://doi.org/10.1016/j.stem.2008.11.006
Nakada, D., Oguro, H., Levi, B. P., Ryan, N., Kitano, A., Saitoh, Y., Takeichi, M., Wendt, G. R., & Morrison, S. J. (2014). Oestrogen increases haematopoietic stem-cell self-renewal in females and during pregnancy. Nature, 505, 555–558. https://doi.org/10.1038/nature12932
Novoszel, P., Drobits, B., Holcmann, M., Fernandes, C. S., Tschismarov, R., Derdak, S., Decker, T., Wagner, E. F., & Sibilia, M. (2021). The AP-1 transcription factors c-Jun and JunB are essential for CD8alpha conventional dendritic cell identity. Cell Death and Differentiation, 28, 2404–2420. https://doi.org/10.1038/s41418-021-00765-4
Funding
This study was supported by National Key Research and Development Project (2019YFA0111800), Major Research Plan of National Natural Science Foundation of China (92268116, 91957107), General Program of National Natural Science Foundation of China (81970095) to BG, and by Innovative Research Team of High-Level Local Universities in Shanghai (SHSMU-ZDCX20212000). We thank the support from Shanghai Frontiers Science Center of Cellular Homeostasis and Human Diseases, and thank Flow Cytometry and Animal Core Facilities of Shanghai Jiao Tong University School of Medicine for the technical support.
Author information
Authors and Affiliations
Contributions
BG, HW and YC conceived the research, designed and supervised the experiments, interpreted data and wrote the manuscript. LL, JH and SZ performed most of the experiments, analyzed data and wrote the manuscript. CY prepared samples for immune-staining analysis.
Corresponding authors
Ethics declarations
Ethics Approval (Include Appropriate Approvals or Waivers)
All animal experiments followed protocols approved by The Institutional Animal Care and Use Committee of Shanghai Jiao Tong University School of Medicine.
Consent to Participate (Include Appropriate Statements)
Not applicable.
Consent for Publication (Include Appropriate Statements)
The authors are alone responsible for the content and writing of the paper. All authors reviewed and approved the final version of the manuscript.
Conflicts of Interest/Competing Interests (Include Appropriate Disclosures)
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
12015_2023_10670_MOESM1_ESM.pdf
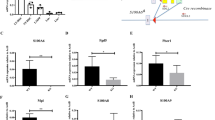
Supplementary file1 Supplemental Figure 1. The CRUs was significantly decreased in the BM of Cct5-/- mice. (A) CRUs in the BM of Cct5fl/fl and Cct5-/- mice, as determined by LDA. (B) The summary chart showing the percentage of recipient mice with positive engraftment (>1% CD45.2 cell chimerism), as well as the CRUs and Range calculated using ELDA software. (C) The bar graph showing the CRUs in 106 BM cells of Cct5fl/fl and Cct5-/- mice. Poisson statistical analysis, ***p < 0.001. (PDF 143 kb)
12015_2023_10670_MOESM2_ESM.pdf
Supplementary file2 Supplemental Figure 2. Knockdown of CCT5 impairs the engraftment of human cord blood HSPCs. (A) Experimental schematics for the transplantation experiments of Control shRNA and CCT5 shRNA transduced cord blood CD34+ cells. (B) Representative FACS plot showing human CD45 chimerism in the BM of recipient immune-deficient mice transplanted with Control shRNA and CCT5 shRNA infected CD34+ cells. (C) Quantification data showing the percentage of human CD45 chimerism in the BM of recipient mice transplanted with Control shRNA and CCT5 shRNA infected CD34+ cells. ***p < 0.001. (D-E) Quantification data showing the percentage of human myeloid and B cell chimerism in the BM of recipient mice transplanted with Control shRNA and CCT5 shRNA infected CD34+ cells. ***p < 0.001. (PDF 388 kb)
Supplemental Table 1.
Protein-protein interaction (PPI) analysis for CCT5/Cct5 using data sourced from the BioGRID and IntAct databases (XLSX 280 kb)
Supplemental Table 2.
Identification of Cct5-interacting TFs using the TFDB database and their downstream target genes (XLSX 2.93 mb)
Supplemental Table 3.
The shared functions between Cct5-TF targets (PPI) and downregulated genes in Cct5 knockout HSPCs (RNA-seq) (XLSX 416 kb)
Supplemental Table 4.
Gene set enrichment analysis (GSEA) showing the down-regulation of genes involved in autophagy, myeloid cell development and actin filament organization in Cct5 knockout HSPCs (XLSX 6.90 mb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, L., Huang, J., Zhang, S. et al. The Chaperone Protein Cct5 is Essential for Hematopoietic Stem Cell Maintenance. Stem Cell Rev and Rep 20, 769–778 (2024). https://doi.org/10.1007/s12015-023-10670-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10670-7