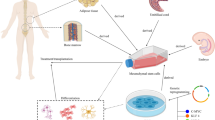
Abstract
Background
Neural stem/progenitor cell (NSPC) transplantation in spinal cord injury (SCI) is a potential treatment that supports regeneration by promoting neuroprotection, remyelination, and neurite outgrowth. However, glial scarring hinders neuroregeneration and reduces the efficiency of cell transplantation. The present study aimed to enhance this neuroregeneration by surgically removing the glial scar and transplanting heat-shock (HS) preconditioned NSPCs in combination with Arg-Gly-Asp (RGD)-functionalised hydrogel in a rat spinal cord hemi-transection model.
Methods
Twelve Sprague-Dawley rats underwent spinal cord hemi-transection and were randomly divided into three treatment groups: hydrogel implantation (control group), NSPC-encapsulated hydrogel implantation, and HS-NSPC-encapsulated hydrogel implantation. HS preconditioning was applied to the NSPCs to reinforce cell retention and an RGD-functionalised hydrogel was used as a biomatrix.
Results
In vitro culture showed that preconditioned NSPCs highly differentiated into neurons and oligodendrocytes and exhibited higher proliferation and neurite outgrowth in hydrogels. Rats in the HS-NSPC-encapsulated hydrogel implantation group showed significantly improved functional recovery, neuronal and oligodendrocyte differentiation of transplanted cells, remyelination, and low fibrotic scar formation.
Conclusions
The surgical removal of the glial scar in combination with HS-preconditioning and RGD-functionalised hydrogels should be considered as a new paradigm in NSPC transplantation for spinal cord regeneration treatment.
Graphical Abstract








Similar content being viewed by others
Data Availability
All data generated or analysed during this study are included in this published article.
Abbreviations
- 3D :
-
Three dimensional
- BBB :
-
Basso, Beattie, Bresnahan Locomotor Rating Scale
- BDNF :
-
Brain-derived neurotrophic factor
- CNS :
-
Central nervous system
- CXCR4 :
-
C-X-C chemokine receptor type 4
- DAPI :
-
4,6-diamidino-2-phenylindole
- FBS :
-
Fetal bovine serum
- ECM :
-
Extracellular matrix
- GAPHD :
-
Glyceraldehyde-3-phosphate dehydrogenase
- GFAP :
-
Glial fibrillary acidic protein
- HG :
-
Hydrogel
- HS :
-
Heat-shock
- HSP27 :
-
Heat-shock protein 70
- HSP70 :
-
Heat-shock protein 70
- LFB :
-
Luxol fast blue
- MBP :
-
Myelin basic protein
- NGF :
-
Nerve growth factor
- NSPC :
-
Neural stem/progenitor cell
- NSC :
-
Neural stem cells
- OCT :
-
Optimal cutting temperature compound
- PBS :
-
Phosphate-buffered saline
- PFA :
-
Paraformaldehyde
- PLO :
-
Poly-L-ornithine solution
- RGD :
-
Arg-Gly-Asp
- RT :
-
Room temperature
- RT-PCR :
-
Real-time polymerase chain reaction
- SCI :
-
Spinal cord injury
- SOX2 :
-
Sex determining region Y-box 2
- VEGF :
-
Vascular endothelial growth factor
References
Bickenbach, J., Boldt, I., Brinkhof, M., Chamberlain, J., Cripps, R., & Fitzharris, M., et al. (2013). A global picture of spinal cord injury. In J. Bickenbach, C. Bodine, D. Brown, A. Burns, R. Campbell, & D. Cardenas (Eds.), International Perspectives on spinal cord Injury (pp. 11–42). World Health Organization.
Hagen, E. M., Rekand, T., Gilhus, N. E., & Grønning, M. (2012). Traumatic spinal cord injuries–incidence, mechanisms and course. Tidsskrift for Den Norske Laegeforening, 132, 831–837.
Silva, N. A., Sousa, N., Reis, R. L., & Salgado, A. J. (2014). From basics to clinical: A comprehensive review on spinal cord injury. Progress in Neurobiology, 114, 25–57.
Gilbert, R. J., McKeon, R. J., Darr, A., Calabro, A., Hascall, V. C., & Bellamkonda, R. V. (2005). CS-4, 6 is differentially upregulated in glial scar and is a potent inhibitor of neurite extension. Molecular and Cellular Neuroscience, 29, 45–558.
Fawcett, J. W. (2006). Overcoming inhibition in the damaged spinal cord. Journal of Neurotrauma, 23, 371–383.
Huang, L., Fu, C., Xiong, F., He, C., & Wei, Q. (2021). Stem cell therapy for spinal cord injury. Cell Transplantation, 30, 096368972198926.
Weiss, S., Dunne, C., Hewson, J., Wohl, C., Wheatley, M., Peterson, A. C., Weiss, S., Dunne, C., Hewson, J., Wohl, C., Wheatley, M., Peterson, A. C., & Reynolds, B. A. (1996). Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. Journal of Neuroscience, 16, 7599–7609.
Yang, J., Jiang, Z., Fitzgerald, D. C., Ma, C., Yu, S., Li, H., Yang, J., Jiang, Z., Fitzgerald, D. C., Ma, C., Yu, S., Li, H., Zhao, Z., Li, Y., Ciric, B., Curtis, M., Rostami, A., & Zhang, G.-X. (2009). Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. The Journal of Clinical Investigation, 119(12), 3678–3691.
Zhu, Y., Uezono, N., Yasui, T., & Nakashima, K. (2018). Neural stem cell therapy aiming at better functional recovery after spinal cord injury. Developmental Dynamics, 247(1), 75–84.
Assunção-Silva, R. C., Gomes, E. D., Sousa, N., Silva, N. A., & Salgado, A. J. (2015). Hydrogels and cell based therapies in spinal cord injury regeneration. Stem Cells International, 2015, 1–24.
Austin, J. W., Kang, C. E., Baumann, M. D., DiDiodato, L., Satkunendrarajah, K., Wilson, J. R., et al. (2012). The effects of intrathecal injection of a hyaluronan-based hydrogel on inflammation, scarring and neurobehavioural outcomes in a rat model of severe spinal cord injury associated with arachnoiditis. Biomaterials, 33, 4555–4564.
Li, G., Che, M. T., Zhang, K., Qin, L. N., Zhang, Y. T., Chen, R. Q., Li, Ge., Che, M.-T., Zhang, Ke., Qin, L.-N., Zhang, Y.-T., Chen, R.-Q., Rong, L.-M., Liu, S., Ding, Y., Shen, H.-Y., Long, S.-M., Wu, J.-L., Ling, E.-A., & Zeng, Y.-S. (2016). Graft of the NT-3 persistent delivery gelatin sponge scaffold promotes axon regeneration, attenuates inflammation, and induces cell migration in rat and canine with spinal cord injury. Biomaterials, 83, 233–248.
Dutta, R. C., & Dutta, A. K. (2009). Cell-interactive 3D-scaffold; advances and applications. Biotechnology Advances, 27, 334–339.
Chan, G., & Mooney, D. J. (2008). New materials for tissue engineering: Towards greater control over the biological response. Trends in Biotechnology, 26, 382–392.
Straley, K. S., Foo, C. W. P., & Heilshorn, S. C. (2010). Biomaterial design strategies for the treatment of spinal cord injuries. Journal of Neurotrauma, 27, 1–19.
Wang, L. S., Chung, J. E., Chan, P. P., & Kurisawa, M. (2010). Injectable biodegradable hydrogels with tunable mechanical properties for the stimulation of neurogenesic differentiation of human mesenchymal stem cells in 3D culture. Biomaterials, 31, 1148–1157.
Tam, R. Y., Cooke, M. J., & Shoichet, M. S. (2012). A covalently modified hydrogel blend of hyaluronan–methyl cellulose with peptides and growth factors influences neural stem/progenitor cell fate. Journal of Materials Chemistry, 22, 19402–19411.
Sakata, H., Niizuma, K., Wakai, T., Narasimhan, P., Maier, C. M., & Chan, P. H. (2012). Neural stem cells genetically modified to overexpress cu/zn-superoxide dismutase enhance amelioration of ischemic stroke in mice. Stroke, 43, 2423–2429.
Lan, F., Liu, J., Narsinh, K. H., Hu, S., Han, L., Lee, A. S., et al. (2012). Safe genetic modification of cardiac stem cells using a site-specific integration technique. Circulation, 126, S20–S28.
Morange, M. (2005). What history tells us II. The discovery of chaperone function. Journal of Biosciences, 30, 461–464.
Robinson, M. B., Tidwell, J. L., Gould, T., Taylor, A. R., Newbern, J. M., Graves, J., Robinson, M. B., Tidwell, J. L., Gould, T., Taylor, A. R., Newbern, J. M., Graves, J., Tytell, M., & Milligan, C. E. (2005). Extracellular heat shock protein 70: A critical component for motoneuron survival. Journal of Neuroscience, 25, 9735–9745.
Chen, Y., Voegeli, T. S., Liu, P. P., Noble, E. G., & Currie, R. W. (2007). Heat shock paradox and a new role of heat shock proteins and their receptors as anti-inflammation targets. Inflammation & Allergy: Drug Targets, 6, 91–100.
Zhou, Y., Xu, L., Song, X., Ding, L., Chen, J., Wang, C., Zhou, Y., Xu, L., Song, X., Ding, L., Chen, J., Wang, C., Gan, Y., Zhu, X., Yu, Y., & Liang, Q. (2014). The potential role of heat shock proteins in acute spinal cord injury. European Spine Journal, 23, 1480–1490.
Kim, W. K., Kim, W. H., Kweon, O. K., & Kang, B. J. (2022). Heat-shock proteins can potentiate the therapeutic ability of Cryopreserved Mesenchymal Stem cells for the treatment of Acute spinal cord Injury in Dogs. Stem Cell Reviews and Report, 18, 1461–1477.
Dasari, V. R., Veeravalli, K. K., & Dinh, D. H. (2014). Mesenchymal stem cells in the treatment of spinal cord injuries: A review. World Journal of Stem Cells, 6(2), 120.
Pekny, M., & Nilsson, M. (2005). Astrocyte activation and reactive gliosis. Glia, 50(4), 427–434.
Shaik, S., Hayes, D., Gimble, J., & Devireddy, R. (2017). Inducing heat shock proteins enhances the stemness of frozen–thawed adipose tissue-derived stem cells. Stem Cells and Development, 26, 608–616.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2 – ∆∆CT method. Methods, 25, 402–408.
Faul, F., Erdfelder, E., Buchner, A., & Lang, A. G. (2009). Statistical power analyses using G* power 3.1: Tests for correlation and regression analyses. Behavior Research Methods, 41(4), 1149–1160.
Abbaszadeh, H. A., Niknazar, S., Darabi, S., Roozbahany, N. A., Noori-Zadeh, A., Ghoreishi, S. K., Abbaszadeh, H.-A., Niknazar, S., Darabi, S., Ahmady Roozbahany, N., Noori-Zadeh, A., Ghoreishi, S. K., Khoramgah, M. S., & Sadeghi, Y. (2018). Stem cell transplantation and functional recovery after spinal cord injury: A systematic review and meta-analysis. Anatomy and Cell Biology, 51(3), 180–188.
Lin, X. J., Wen, S., Deng, L. X., Dai, H., Du, X., Chen, C., et al. (2020). Spinal cord lateral hemisection and asymmetric behavioral assessments in adult rats. Journal of Visualized Experiments: Jove, 157, e57126.
Chen, Z. L., Yu, W. M., & Strickland, S. (2007). Peripheral regeneration. Annual Review of Neuroscience, 30, 209–233.
Battersby, A., Jones, R. D., Lilley, K. S., McFarlane, R. J., Braig, H. R., Allen, N. D., Battersby, A., Jones, R. D., Lilley, K. S., McFarlane, R. J., Braig, H. R., Allen, N. D., & Wakeman, J. A. (2007). Comparative proteomic analysis reveals differential expression of Hsp25 following the directed differentiation of mouse embryonic stem cells. Biochimica Et Biophysica Acta, 1773, 147–156.
Chen, J., Crawford, R., Chen, C., & Xiao, Y. (2013). The key regulatory roles of the PI3K/Akt signaling pathway in the functionalities of mesenchymal stem cells and applications in tissue regeneration. Tissue Engineering. Part B, Reviews, 19(6), 516–528.
Gao, F., Hu, X. Y., Xie, X. J., Xu, Q. Y., Wang, Y. P., Liu, X. B., Gao, F., Hu, X.-Y., Xie, X.-J., Xu, Q.-Y., Wang, Y.-P., Liu, X.-B., Xiang, M.-X., Sun, Y., & Wang, J.-a. (2010). Heat shock protein 90 protects rat mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis via the PI3K/Akt and ERK1/2 pathways. Journal of Zhejiang University. Science. B, 11, 608–617.
Friedman, W. J., Thakur, S., Seidman, L., & Rabson, A. B. (1996). Regulation of nerve growth factor mRNA by interleukin-1 in rat hippocampal astrocytes is mediated by NFκB. Journal of Biological Chemistry, 271, 31115–31120.
Lipsky, R. H., Xu, K., Zhu, D., Kelly, C., Terhakopian, A., Novelli, A., et al. (2001). Nuclear factor κB is a critical determinant in N-methyl‐d‐aspartate receptor‐mediated neuroprotection. Journal of Neurochemistry, 78, 254–264.
Sart, S., Tsai, A. C., Li, Y., & Ma, T. (2014). Three-dimensional aggregates of mesenchymal stem cells: Cellular mechanisms, biological properties, and applications. Tissue Engineering. Part B, Reviews, 20, 365–380.
Ye, Z., Zhou, Y., Cai, H., & Tan, W. (2011). Myocardial regeneration: Roles of stem cells and hydrogels. Advanced Drug Delivery Reviews, 63, 688–697.
Baharvand, H., Fathi, A., van Hoof, D., & Salekdeh, G. H. (2007). Concise review: Trends in stem cell proteomics. Stem Cells, 25, 1888–1903.
Read, D. E., & Gorman, A. M. (2009). Heat shock protein 27 in neuronal survival and neurite outgrowth. Biochemical and Biophysical Research Communications, 382, 6–8.
Lu, P., Wang, Y., Graham, L., McHale, K., Gao, M., Wu, D., Lu, P., Wang, Y., Graham, L., McHale, K., Gao, M., Wu, Di., Brock, J., Blesch, A., Rosenzweig, E., Havton, L., Zheng, B., Conner, J., Marsala, M., & Tuszynski, M. (2012). Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell, 150, 1264–1273.
Ek, C. J., Habgood, M. D., Callaway, J. K., Dennis, R., Dziegielewska, K. M., Johansson, P. A., et al. (2010). Spatio-temporal progression of grey and white matter damage following contusion injury in rat spinal cord. PLoS One, 5(8), e12021.
Peng, H., Kolb, R., Kennedy, J. E., & Zheng, J. (2007). Differential expression of CXCL12 and CXCR4 during human fetal neural progenitor cell differentiation. Journal of Neuroimmune Pharmacology: The Official Journal of the Society on Neuroimmune Pharmacology, 2, 251–258.
Orr, M. B., & Gensel, J. C. (2018). Spinal cord injury scarring and inflammation: Therapies targeting glial and inflammatory responses. Neurotherapeutics, 15, 541–553.
Amor, S., & Woodroofe, M. N. (2014). Innate and adaptive immune responses in neurodegeneration and repair. Immunology, 141, 287–291.
Chen, L. W., Kuang, F., Wei, L. C., Ding, Y. X., Yung, K. K., & Chan, Y. S. (2011). Potential application of induced pluripotent stem cells in cell replacement therapy for Parkinson’s disease. CNS & Neurological Disorders: Drug Targets, 10, 449–458.
Tsuji, O., Miura, K., Okada, Y., Fujiyoshi, K., Mukaino, M., Nagoshi, N., Tsuji, O., Miura, K., Okada, Y., Fujiyoshi, K., Mukaino, M., Nagoshi, N., Kitamura, K., Kumagai, G., Nishino, M., Tomisato, S., Higashi, H., Nagai, T., Katoh, H., … Okano, H. (2010). Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proceedings National Academy of Sciences U S A, 107, 12704–12709.
Willis, C. M., Nicaise, A. M., Peruzzotti-Jametti, L., & Pluchino, S. (2020). The neural stem cell secretome and its role in brain repair. Brain Research, 1729, 146615.
Campisi, J., & Fleshner, M. (2003). Role of extracellular HSP72 in acute stress-induced potentiation of innate immunity in active rats. Journal of Applied Physiology, 94, 43–52.
Neumann, H. (2000). The immunological microenvironment in the CNS: Implications on neuronal cell death and survival. Journal of Neural Transmission. Supplementum, 59, 59–68.
Kim, H., Cooke, M. J., & Shoichet, M. S. (2012). Creating permissive microenvironments for stem cell transplantation into the central nervous system. Trends in Biotechnology, 30, 55–63.
Nicaise, A. M., D’Angelo, A., Ionescu, R. B., Krzak, G., Willis, C. M., & Pluchino, S. (2022). The role of neural stem cells in regulating glial scar formation and repair. Cell and Tissue Research, 387, 399–414.
Tseng, T. C., Tao, L., Hsieh, F. Y., Wei, Y., Chiu, I. M., & Hsu, S. H. (2015). An injectable, self-healing hydrogel to repair the central nervous system. Advanced Materials, 27, 3518–3524.
Banerjee, A., Arha, M., Choudhary, S., Ashton, R. S., Bhatia, S. R., Schaffer, D. V., Banerjee, A., Arha, M., Choudhary, S., Ashton, R. S., Bhatia, S. R., Schaffer, D. V., & Kane, R. S. (2009). The influence of hydrogel modulus on the proliferation and differentiation of encapsulated neural stem cells. Biomaterials, 30, 4695–4699.
Haruna, N., & Huang, J. (2020). Investigating the dynamic Biophysical Properties of a tunable hydrogel for 3D cell culture. Journal of Cytology Tissue Biology, 7, 030.
Tajiri, N., Kaneko, Y., Shinozuka, K., Ishikawa, H., Yankee, E., McGrogan, M., Tajiri, N., Kaneko, Y., Shinozuka, K., Ishikawa, H., Yankee, E., McGrogan, M., Case, C., & Borlongan, C. V. (2013). Stem cell recruitment of newly formed host cells via a successful seduction? Filling the gap between neurogenic niche and injured brain site. PLoS One, 8, e74857.
Poplawski, G. H. D., Kawaguchi, R., Van Niekerk, E., Lu, P., Mehta, N., Canete, P., Poplawski, G. H. D., Kawaguchi, R., Van Niekerk, E., Lu, P., Mehta, N., Canete, P., Lie, R., Dragatsis, I., Meves, J. M., Zheng, B., Coppola, G., & Tuszynski, M. H. (2020). Injured adult neurons regress to an embryonic transcriptional growth state. Nature, 581, 77–82.
Han, Q., Xie, Y., Ordaz, J. D., Huh, A. J., Huang, N., Wu, W., et al. (2020). Restoring cellular energetics promotes axonal regeneration and functional recovery after spinal cord injury. Cell Metabolism, 31, 623–41e8.
Marín, O., Valiente, M., Ge, X., & Tsai, L. H. (2010). Guiding neuronal cell migrations. Cold Spring Harbor Perspectives in Biology, 2, a001834.
Moraga, A., Pradillo, J. M., Cuartero, M. I., Hernández-Jiménez, M., Oses, M., Moro, M. A., et al. (2014). Toll-like receptor 4 modulates cell migration and cortical neurogenesis after focal cerebral ischemia. The Faseb Journal, 28, 4710–4718.
DeGeer, J., Kaplan, A., Mattar, P., Morabito, M., Stochaj, U., Kennedy, T. E., DeGeer, J., Kaplan, A., Mattar, P., Morabito, M., Stochaj, U., Kennedy, T. E., Debant, A., Cayouette, M., Fournier, A. E., & Lamarche-Vane, N. (2015). Hsc70 chaperone activity underlies Trio GEF function in axon growth and guidance induced by netrin-1. Journal of Cell Biology, 210, 817–832.
Acknowledgements
We thank all the members who contributed to this article.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (No. 2018R1D1A1B07047451). The funding body played no role in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Author information
Authors and Affiliations
Contributions
WKK was involved in study design and performed experiments, data analysis, and manuscript writing. BJK were involved in study design and revised the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
The study was approved by the Animal Care and Use Committee of Seoul National University (SNU-220103-6-1; Title: Development of spinal cord injury treatment using neural stem/progenitor cells; Date of approval: April 13, 2022).
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, W.K., Kang, BJ. Transplantation of Heat-Shock Preconditioned Neural Stem/Progenitor Cells Combined with RGD-Functionalised Hydrogel Promotes Spinal Cord Functional Recovery in a Rat Hemi-Transection Model. Stem Cell Rev and Rep 20, 283–300 (2024). https://doi.org/10.1007/s12015-023-10637-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10637-8