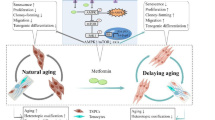
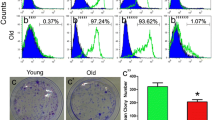
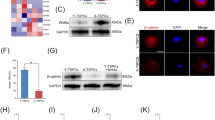
Abstract
Age-related tendon disorder, a primary motor system disease, is characterized by biological changes in the tendon tissue due to senescence and seriously affects the quality of life of the elderly. The pathogenesis of this disease is not well-understood. Tendon stem/progenitor cells (TSPCs) exhibit multi-differentiation capacity. These cells are important cellular components of the tendon because of their roles in tendon tissue homeostasis, remodeling, and repair. Previous studies revealed alterations in the biological characteristics and tenogenic differentiation potential of TSPCs in senescent tendon tissue, in turn contributing to insufficient differentiation of TSPCs into tenocytes. Poor tendon repair can result in age-related tendinopathies. Therefore, targeting of senescent TSPCs may restore the tenogenic differentiation potential of these cells and achieve homeostasis of the tendon tissue to prevent or treat age-related tendinopathy. In this review, we summarize the biological characteristics of TSPCs and histopathological changes in age-related tendinopathy, as well as the potential mechanisms through which TSPCs contribute to senescence. This information may promote further exploration of innovative treatment strategies to rescue TSPCs from senescence.


Similar content being viewed by others
Data Availability
Not applicable.
References
Fuellen, G., et al. (2019). Health and Aging: unifying concepts, scores, biomarkers and pathways. Aging Dis, 10, 883–900.
Li, Y., et al. (2019). The potential roles of Tendon Stem/Progenitor cells in Tendon Aging. Curr Stem Cell Res Ther, 14, 34–42.
Zhang, Y. W., et al. (2022). Fecal microbiota transplantation ameliorates bone loss in mice with ovariectomy-induced osteoporosis via modulating gut microbiota and metabolic function. J Orthop Translat, 37, 46–60.
Almekinders, L. C., & Deol, G. (1999). The effects of aging, antiinflammatory drugs, and ultrasound on the in vitro response of tendon tissue. American Journal Of Sports Medicine, 27, 417–421.
Zhang, Y. W., et al. (2022). The regulative effect and repercussion of probiotics and prebiotics on osteoporosis: involvement of brain-gut-bone axis. Crit Rev Food Sci Nutr, 1–19
Zhang, X., et al. (2022). Clinical perspectives for repairing rotator cuff injuries with multi-tissue regenerative approaches. J Orthop Translat, 36, 91–108.
Lui, P. P. Y., & Wong, C. M. (2019). Biology of Tendon Stem cells and Tendon in Aging. Frontiers In Genetics, 10, 1338.
Birch, H. L., Peffers, M. J., & Clegg, P. D. (2016). Influence of Ageing on Tendon Homeostasis. Advances In Experimental Medicine And Biology, 920, 247–260.
Zhou, B., Zhou, Y., & Tang, K. (2014). An overview of structure, mechanical properties, and treatment for age-related tendinopathy. The Journal Of Nutrition, Health & Aging, 18, 441–448.
Dunkman, A. A., et al. (2013). Decorin expression is important for age-related changes in tendon structure and mechanical properties. Matrix Biology, 32, 3–13.
Gehwolf, R., et al. (2016). Pleiotropic roles of the matricellular protein Sparc in tendon maturation and ageing. Scientific Reports, 6, 32635.
Delabastita, T., Bogaerts, S., & Vanwanseele, B. (2018). Age-Related Changes in Achilles Tendon Stiffness and Impact on Functional Activities: A Systematic Review and Meta-Analysis. J Aging Phys Act, 1–12
Kostrominova, T. Y., & Brooks, S. V. (2013). Age-related changes in structure and extracellular matrix protein expression levels in rat tendons. Age (Dordr), 35, 2203–2214.
Arnesen, S. M., & Lawson, M. A. (2006). Age-related changes in focal adhesions lead to altered cell behavior in tendon fibroblasts. Mechanisms Of Ageing And Development, 127, 726–732.
Rui, Y. F., et al. (2011). Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. Journal Of Orthopaedic Research, 29, 390–396.
Yu, T. Y., et al. (2013). Aging is associated with increased activities of matrix metalloproteinase-2 and – 9 in tenocytes. Bmc Musculoskeletal Disorders, 14, 2.
Rui, Y. F., Lui, P. P., Wong, Y. M., Tan, Q., & Chan, K. M. (2013). BMP-2 stimulated non-tenogenic differentiation and promoted proteoglycan deposition of tendon-derived stem cells (TDSCs) in vitro. Journal Of Orthopaedic Research, 31, 746–753.
Rui, Y. F., Lui, P. P., Wong, Y. M., Tan, Q., & Chan, K. M. (2013). Altered fate of tendon-derived stem cells isolated from a failed tendon-healing animal model of tendinopathy. Stem Cells And Development, 22, 1076–1085.
Jain, N. B., et al. (2019). Operative vs nonoperative treatment for atraumatic rotator cuff tears: a Trial Protocol for the arthroscopic rotator cuff pragmatic randomized clinical trial. JAMA Netw Open, 2, e199050.
Bechay, J., Lawrence, C., & Namdari, S. (2020). Calcific tendinopathy of the rotator cuff: a review of operative versus nonoperative management. Phys Sportsmed, 48, 241–246.
Xu, K., et al. (2021). Spironolactone Ameliorates Senescence and Calcification by Modulating Autophagy in Rat Tendon-Derived Stem Cells via the NF-kappaB/MAPK Pathway. Oxid Med Cell Longev 5519587 (2021).
Zaseck, L. W., Miller, R. A., & Brooks, S. V. (2016). Rapamycin attenuates Age-associated changes in Tibialis Anterior Tendon Viscoelastic Properties. Journals Of Gerontology. Series A, Biological Sciences And Medical Sciences, 71, 858–865.
Bi, Y., et al. (2007). Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nature Medicine, 13, 1219–1227.
Rajpar, I., & Barrett, J. G. (2020). Multi-differentiation potential is necessary for optimal tenogenesis of tendon stem cells. Stem Cell Research & Therapy, 11, 152.
Wang, J. H., & Komatsu, I. (2016). Tendon Stem cells: Mechanobiology and Development of Tendinopathy. Advances In Experimental Medicine And Biology, 920, 53–62.
Kim, S. J., Song, D. H., & Kim, S. J. (2018). Characteristics of tendon derived stem cells according to different factors to induce the tendinopathy. Journal Of Cellular Physiology, 233, 6196–6206.
Rui, Y. F., et al. (2012). Expression of chondro-osteogenic BMPs in clinical samples of patellar tendinopathy. Knee Surgery, Sports Traumatology, Arthroscopy, 20, 1409–1417.
Rui, Y. F., et al. (2011). Does erroneous differentiation of tendon-derived stem cells contribute to the pathogenesis of calcifying tendinopathy? Chin Med J (Engl), 124, 606–610.
Zhou, Z., et al. (2010). Tendon-derived stem/progenitor cell aging: defective self-renewal and altered fate. Aging Cell, 9, 911–915.
Kohler, J., et al. (2013). Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell, 12, 988–999.
Zhang, J., & Wang, J. H. (2010). Mechanobiological response of tendon stem cells: implications of tendon homeostasis and pathogenesis of tendinopathy. Journal Of Orthopaedic Research, 28, 639–643.
Wood, L. K., & Brooks, S. V. (2016). Ten weeks of treadmill running decreases stiffness and increases collagen turnover in tendons of old mice. Journal Of Orthopaedic Research, 34, 346–353.
Dai, G., et al. (2020). Higher BMP expression in Tendon Stem/Progenitor cells contributes to the increased heterotopic ossification in Achilles Tendon with Aging. Front Cell Dev Biol, 8, 570605.
Wu, H., Zhao, G., Zu, H., Wang, J. H., & Wang, Q. M. (2015). Aging-related viscoelasticity variation of tendon stem cells (TSCs) characterized by quartz thickness shear mode (TSM) resonators. Sens Actuators (Warrendale Pa), 210, 369–380.
Zhang, J., Yuan, T., & Wang, J. H. (2016). Moderate treadmill running exercise prior to tendon injury enhances wound healing in aging rats. Oncotarget, 7, 8498–8512.
Tsai, W. C., et al. (2011). Decreased proliferation of aging tenocytes is associated with down-regulation of cellular senescence-inhibited gene and up-regulation of p27. Journal Of Orthopaedic Research, 29, 1598–1603.
Verzijl, N., et al. (2000). Effect of collagen turnover on the accumulation of advanced glycation end products. Journal Of Biological Chemistry, 275, 39027–39031.
Shinohara, I., et al. (2022). Biochemical markers of aging (Advanced Glycation End Products) and degeneration are increased in type 3 Rotator Cuff Tendon Stumps with increased Signal Intensity Changes on MRI. American Journal Of Sports Medicine, 50, 1960–1970.
Dai, J., Chen, H., & Chai, Y. (2019). Advanced Glycation End Products (AGEs) induce apoptosis of fibroblasts by activation of NLRP3 inflammasome via reactive oxygen species (ROS) signaling pathway. Medical Science Monitor, 25, 7499–7508.
Hegedus, E. J., et al. (2010). Vascularity and tendon pathology in the rotator cuff: a review of literature and implications for rehabilitation and surgery. British Journal Of Sports Medicine, 44, 838–847.
Vailas, A. C., Tipton, C. M., Laughlin, H. L., Tcheng, T. K., & Matthes, R. D. (1978). Physical activity and hypophysectomy on the aerobic capacity of ligaments and tendons. J Appl Physiol Respir Environ Exerc Physiol, 44, 542–546.
Andarawis-Puri, N., Flatow, E. L., & Soslowsky, L. J. (2015). Tendon basic science: development, repair, regeneration, and healing. Journal Of Orthopaedic Research, 33, 780–784.
Tuite, D. J., Renstrom, P. A., & O’Brien, M. (1997). The aging tendon. Scandinavian Journal Of Medicine And Science In Sports, 7, 72–77.
Zhang, X., et al. (2016). Therapeutic Roles of Tendon Stem/Progenitor Cells in Tendinopathy. Stem Cells Int 4076578 (2016).
Prasnikar, E., Borisek, J., & Perdih, A. (2021). Senescent cells as promising targets to tackle age-related diseases. Ageing Research Reviews, 66, 101251.
McHugh, D., & Gil, J. (2018). Senescence and aging: causes, consequences, and therapeutic avenues. Journal Of Cell Biology, 217, 65–77.
Wei, W., & Ji, S. (2018). Cellular senescence: molecular mechanisms and pathogenicity. Journal Of Cellular Physiology, 233, 9121–9135.
Gorgoulis, V., et al. (2019). Cellular Senescence: defining a path Forward. Cell, 179, 813–827.
Dai, G. C., Li, Y. J., Chen, M. H., Lu, P. P., & Rui, Y. F. (2019). Tendon stem/progenitor cell ageing: modulation and rejuvenation. World J Stem Cells, 11, 677–692.
Chen, M., et al. (2020). AQP1 modulates tendon stem/progenitor cells senescence during tendon aging. Cell Death And Disease, 11, 193.
Hu, C., et al. (2017). Downregulation of CITED2 contributes to TGFbeta-mediated senescence of tendon-derived stem cells. Cell And Tissue Research, 368, 93–104.
Xu, H., & Liu, F. (2018). Downregulation of FOXP1 correlates with tendon stem/progenitor cells aging. Biochemical And Biophysical Research Communications, 504, 96–102.
Chen, L., et al. (2015). The role of Pin1 protein in aging of human tendon stem/progenitor cells. Biochemical And Biophysical Research Communications, 464, 487–492.
Herranz, N., & Gil, J. (2018). Mechanisms and functions of cellular senescence. J Clin Invest, 128, 1238–1246.
Chen, M., et al. (2021). Inhibition of JAK-STAT signaling pathway alleviates Age-Related phenotypes in Tendon Stem/Progenitor cells. Front Cell Dev Biol, 9, 650250.
Chen, M., et al. (2021). Noncanonical Wnt5a signaling regulates tendon stem/progenitor cells senescence. Stem Cell Research & Therapy, 12, 544.
Zhang, K., Asai, S., Yu, B., & Enomoto-Iwamoto, M. (2015). IL-1beta irreversibly inhibits tenogenic differentiation and alters metabolism in injured tendon-derived progenitor cells in vitro. Biochemical And Biophysical Research Communications, 463, 667–672.
Chen, S., et al. (2018). Interleukin-6 promotes proliferation but inhibits tenogenic differentiation via the Janus Kinase/Signal Transducers and Activators of transcription 3 (JAK/STAT3) pathway in Tendon-Derived stem cells. Medical Science Monitor, 24, 1567–1573.
Li, G., et al. (2021). Rejuvenation of senescent bone marrow mesenchymal stromal cells by Pulsed Triboelectric Stimulation. Adv Sci (Weinh), 8, e2100964.
Amano, H., et al. (2019). Telomere Dysfunction induces Sirtuin repression that drives Telomere-Dependent Disease. Cell Metab, 29, 1274–1290e1279.
Srinivas, N., Rachakonda, S., & Kumar, R. (2020). Telomeres and Telomere Length: A General Overview. Cancers (Basel)12
Martinez, P., & Blasco, M. A. (2011). Telomeric and extra-telomeric roles for telomerase and the telomere-binding proteins. Nature Reviews Cancer, 11, 161–176.
de Bernardes, B., & Blasco, M. A. (2013). Telomerase at the intersection of cancer and aging. Trends In Genetics, 29, 513–520.
Kauppila, T. E. S., Kauppila, J. H. K., & Larsson, N. G. (2017). Mammalian Mitochondria and Aging: an update. Cell Metab, 25, 57–71.
Chen, H., et al. (2016). Autophagy prevents oxidative Stress-Induced loss of Self-Renewal Capacity and Stemness in Human Tendon Stem cells by reducing ROS Accumulation. Cellular Physiology And Biochemistry, 39, 2227–2238.
Lee, B. Y., et al. (2006). Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell, 5, 187–195.
Hernandez-Segura, A., et al. (2017). Unmasking transcriptional heterogeneity in senescent cells. Current Biology, 27, 2652–2660e2654.
Wang, C., et al. (2022). Inhibition of IKKbeta/NF-kappaB signaling facilitates tendinopathy healing by rejuvenating inflamm-aging induced tendon-derived stem/progenitor cell senescence. Mol Ther Nucleic Acids, 27, 562–576.
Zhang, J., & Wang, J. H. (2015). Moderate Exercise mitigates the detrimental Effects of Aging on Tendon Stem cells. PLoS One, 10, e0130454.
Kiderlen, S., et al. (2019). Age related changes in cell stiffness of tendon stem/progenitor cells and a rejuvenating effect of ROCK-inhibition. Biochemical And Biophysical Research Communications, 509, 839–844.
Yin, H., et al. (2020). Three-dimensional self-assembling nanofiber matrix rejuvenates aged/degenerative human tendon stem/progenitor cells. Biomaterials, 236, 119802.
Yan, Z., et al. (2020). Aged Tendon Stem/Progenitor cells are less competent to form 3D Tendon Organoids due to Cell Autonomous and Matrix Production deficits. Frontiers In Bioengineering And Biotechnology, 8, 406.
Ruzzini, L., et al. (2014). Characterization of age-related changes of tendon stem cells from adult human tendons. Knee Surgery, Sports Traumatology, Arthroscopy, 22, 2856–2866.
Rui, Y. F., et al. (2019). CTGF Attenuates Tendon-Derived Stem/Progenitor Cell Aging. Stem Cells Int 6257537 (2019).
Han, W., Tao, X., Weng, T., Chen, L., & Circular (2022). RNA PVT1 inhibits tendon stem/progenitor cell senescence by sponging microRNA-199a-5p. Toxicology In Vitro, 79, 105297.
Klatte-Schulz, F., et al. (2012). Influence of age on the cell biological characteristics and the stimulation potential of male human tenocyte-like cells. Eur Cell Mater, 24, 74–89.
Alberton, P., et al. (2015). Loss of tenomodulin results in reduced self-renewal and augmented senescence of tendon stem/progenitor cells. Stem Cells And Development, 24, 597–609.
Chen, J., et al. (2016). Characterization and comparison of post-natal rat Achilles tendon-derived stem cells at different development stages. Scientific Reports, 6, 22946.
Chen, L., et al. (2015). miR-135a modulates tendon stem/progenitor cell senescence via suppressing ROCK1. Bone, 71, 210–216.
Nie, D., et al. (2021). Rapamycin Treatment of Tendon Stem/Progenitor Cells Reduces Cellular Senescence by Upregulating Autophagy. Stem Cells Int 6638249 (2021).
Popov, C., Kohler, J., & Docheva, D. (2015). Activation of EphA4 and EphB2 Reverse Signaling restores the Age-Associated reduction of Self-Renewal, Migration, and actin turnover in human tendon Stem/Progenitor cells. Frontiers In Aging Neuroscience, 7, 246.
Jiang, D., Xu, B., & Gao, P. (2018). Effects of young extracellular matrix on the biological characteristics of aged tendon stem cells. Adv Clin Exp Med, 27, 1625–1630.
Han, W., Wang, B., Liu, J., & Chen, L. (2017). The p16/miR-217/EGR1 pathway modulates age-related tenogenic differentiation in tendon stem/progenitor cells. Acta Biochimica Et Biophysica Sinica (Shanghai), 49, 1015–1021.
Tan, Q., Lui, P. P., Rui, Y. F., & Wong, Y. M. (2012). Comparison of potentials of stem cells isolated from tendon and bone marrow for musculoskeletal tissue engineering. Tissue Engineering Part A, 18, 840–851.
Zhang, C., et al. (2018). Histone deacetylase inhibitor treated cell sheet from mouse tendon stem/progenitor cells promotes tendon repair. Biomaterials, 172, 66–82.
Magne, D., & Bougault, C. (2015). What understanding tendon cell differentiation can teach us about pathological tendon ossification. Histology And Histopathology, 30, 901–910.
O’Brien, E. J., Frank, C. B., Shrive, N. G., Hallgrimsson, B., & Hart, D. A. (2012). Heterotopic mineralization (ossification or calcification) in tendinopathy or following surgical tendon trauma. International Journal Of Experimental Pathology, 93, 319–331.
Jiang, D., et al. (2014). Effect of young extrinsic environment stimulated by hypoxia on the function of aged tendon stem cell. Cell Biochemistry And Biophysics, 70, 967–973.
Xu, Y., & Murrell, G. A. (2008). The basic science of tendinopathy. Clinical Orthopaedics And Related Research, 466, 1528–1538.
Schmitz, A. A., Govek, E. E., Bottner, B., & Van Aelst, L. (2000). Rho GTPases: signaling, migration, and invasion. Experimental Cell Research, 261, 1–12.
Kato, J. Y., Matsuoka, M., Polyak, K., Massague, J., & Sherr, C. J. (1994). Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27Kip1) of cyclin-dependent kinase 4 activation. Cell, 79, 487–496.
Istvanffy, R., et al. (2015). Stroma-derived connective tissue growth factor maintains cell cycle progression and repopulation activity of hematopoietic stem cells in Vitro. Stem Cell Reports, 5, 702–715.
Yuda, A., et al. (2015). Effect of CTGF/CCN2 on osteo/cementoblastic and fibroblastic differentiation of a human periodontal ligament stem/progenitor cell line. Journal Of Cellular Physiology, 230, 150–159.
Abdelmohsen, K., & Gorospe, M. (2015). Noncoding RNA control of cellular senescence. Wiley Interdiscip Rev RNA, 6, 615–629.
Arthur, A., et al. (2011). EphB/ephrin-B interactions mediate human MSC attachment, migration and osteochondral differentiation. Bone, 48, 533–542.
Moqbel, S. A. A., et al. (2020). Tectorigenin alleviates inflammation, apoptosis, and ossification in rat tendon-derived stem cells via modulating NF-Kappa B and MAPK pathways. Front Cell Dev Biol, 8, 568894.
Liu, C., et al. (2018). Tendon-Derived Stem Cell Differentiation in the Degenerative Tendon Microenvironment. Stem Cells Int 2613821 (2018).
Zhang, J., & Wang, J. H. (2012). BMP-2 mediates PGE(2) -induced reduction of proliferation and osteogenic differentiation of human tendon stem cells. Journal Of Orthopaedic Research, 30, 47–52.
Ellis, S. J., & Tanentzapf, G. (2010). Integrin-mediated adhesion and stem-cell-niche interactions. Cell And Tissue Research, 339, 121–130.
Liu, C., Arnold, R., Henriques, G., & Djabali, K. (2019). Inhibition of JAK-STAT Signaling with Baricitinib Reduces Inflammation and Improves Cellular Homeostasis in Progeria Cells. Cells 8
Xu, M., et al. (2015). JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age. Proc Natl Acad Sci U S A, 112, E6301–6310.
Baker, D. J., et al. (2011). Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature, 479, 232–236.
Baker, D. J., et al. (2016). Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature, 530, 184–189.
Xu, M., et al. (2015). Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife, 4, e12997.
Ogrodnik, M., et al. (2017). Cellular senescence drives age-dependent hepatic steatosis. Nature Communications, 8, 15691.
Khosla, S., Farr, J. N., & Kirkland, J. L. (2018). Inhibiting Cellular Senescence: a New Therapeutic paradigm for age-related osteoporosis. Journal Of Clinical Endocrinology And Metabolism, 103, 1282–1290.
Farr, J. N., et al. (2017). Targeting cellular senescence prevents age-related bone loss in mice. Nature Medicine, 23, 1072–1079.
Chang, J., et al. (2016). Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nature Medicine, 22, 78–83.
Yosef, R., et al. (2016). Directed elimination of senescent cells by inhibition of BCL-W and BCL-XL. Nature Communications, 7, 11190.
Zhu, Y., et al. (2017). New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463. Aging (Albany NY), 9, 955–963.
Cho, H. H., et al. (2005). Induction of osteogenic differentiation of human mesenchymal stem cells by histone deacetylase inhibitors. Journal Of Cellular Biochemistry, 96, 533–542.
Zhang, Y. W., et al. (2022). A narrative review of the moderating effects and repercussion of exercise intervention on osteoporosis: ingenious involvement of gut microbiota and its metabolites. J Transl Med, 20, 490.
Thampatty, B. P., & Wang, J. H. (2018). Mechanobiology of young and aging tendons: in vivo studies with treadmill running. Journal Of Orthopaedic Research, 36, 557–565.
Burton, D. G., & Krizhanovsky, V. (2014). Physiological and pathological consequences of cellular senescence. Cellular And Molecular Life Sciences, 71, 4373–4386.
Song, P., An, J., & Zou, M. H. (2020). Immune Clearance of Senescent Cells to Combat Ageing and Chronic Diseases. Cells 9
Di Micco, R., Krizhanovsky, V., & Baker, D. (2021). d’Adda di Fagagna, F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nature Reviews Molecular Cell Biology, 22, 75–95.
Kim, K. M., et al. (2017). Identification of senescent cell surface targetable protein DPP4. Genes & Development, 31, 1529–1534.
Sadelain, M., Riviere, I., & Riddell, S. (2017). Therapeutic T cell engineering. Nature, 545, 423–431.
Amor, C., et al. (2020). Senolytic CAR T cells reverse senescence-associated pathologies. Nature, 583, 127–132.
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126, 663–676.
Lapasset, L., et al. (2011). Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes & Development, 25, 2248–2253.
Ocampo, A., et al. (2016). Vivo amelioration of Age-Associated. Hallmarks by Partial Reprogramming Cell, 167, 1719–1733e1712.
Kim, S. J., Oh, H. W., Chang, J. W., & Kim, S. J. (2020). Recovery of Tendon Characteristics by Inhibition of Aberrant Differentiation of Tendon-Derived Stem Cells from Degenerative Tendinopathy. Int J Mol Sci 21
Acknowledgements
Not applicable.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81572187 and No.81871812); Natural Science Foundation of Jiangsu Province (BK20221462).
Author information
Authors and Affiliations
Contributions
Wang H and Dai GC completed most of the writing; Li YJ and Zhang M provided assistance with the writing and searched for relevant publications; Chen MH and Zhang YW prepared the figure; Lu PP and Cao MM provided input during drafting of the paper; Rui YF conceived the idea, revised and proofread the paper.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Institutional Review Board
Not applicable.
Informed Consent
Not applicable.
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, H., Dai, GC., Li, YJ. et al. Targeting Senescent Tendon Stem/Progenitor Cells to Prevent or Treat Age-Related Tendon Disorders. Stem Cell Rev and Rep 19, 680–693 (2023). https://doi.org/10.1007/s12015-022-10488-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10488-9