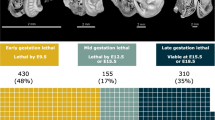
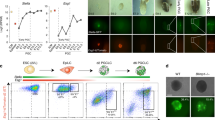
Abstract
By virtue of its inaccessible nature, mammalian implantation stage development has remained one of the most enigmatic and hard to investigate periods of embryogenesis. Derived from pluripotent stem cells, gastruloids recapitulate key aspects of gastrula-stage embryos and have emerged as a powerful in vitro tool to study the architectural features of early post-implantation embryos. While the majority of the work in this emerging field has focused on the use of gastruloids to model embryogenesis, their tractable nature and suitability for high-throughput scaling, has presented an unprecedented opportunity to investigate both developmental and environmental aberrations to the embryo as they occur in vitro. This review summarises the recent developments in the use of gastruloids to model congenital anomalies, their usage in teratogenicity testing, and the current limitations of this emerging field.
Graphical Abstract



Similar content being viewed by others
Data Availability
All data as part of this study are included in this published article.
Code Availability
Not applicable.
References
Macklon, N. S., et al. (2002). Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Human Reproduction Update, 8(4), 333–343
Jenkinson, E. J., & Wilson, I. B. (1970). In vitro support system for the study of blastocyst differentiation in the mouse. Nature, 228(5273), 776–778
Hsu, Y. C. (1971). Post-blastocyst differentiation in vitro. Nature, 231(5298), 100–102
Hsu, Y. C. (1972). Differentiation in vitro of mouse embryos beyond the implantation stage. Nature, 239(5369), 200–202
Pienkowski, M., et al. (1974). Early mouse embryos: growth and differentiation in vitro. Experimental Cell Research, 85(2), 424–428
Wilson, I. B., & Jenkinson, E. J. (1974). Blastocyst differentiation in vitro. J Reprod Fertil, 39(1), 243–249
Wiley, L. M., & Pedersen, R. A. (1977). Morphology of mouse egg cylinder development in vitro: a light and electron microscopic study. Journal Of Experimental Zoology, 200(3), 389–402
Copp, A. J. (1981). The mechanism of mouse egg-cylinder morphogenesis in vitro. Journal Of Embryology And Experimental Morphology, 61, 277–287
Li, S., et al. (2003). The role of laminin in embryonic cell polarization and tissue organization. Developmental Cell, 4(5), 613–624
Bedzhov, I., et al. (2014). In vitro culture of mouse blastocysts beyond the implantation stages. Nature Protocols, 9(12), 2732–2739
Bedzhov, I., & Zernicka-Goetz, M. (2014). Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation. Cell, 156(5), 1032–1044
Morris, S. A., et al. (2012). Dynamics of anterior-posterior axis formation in the developing mouse embryo. Nature Communications, 3, 673
van den Brink, S. C., et al. (2014). Symmetry breaking, germ layer specification and axial organisation in aggregates of mouse embryonic stem cells. Development, 141(22), 4231–4242
Turner, D. A., et al. (2017). Anteroposterior polarity and elongation in the absence of extra-embryonic tissues and of spatially localised signalling in gastruloids: mammalian embryonic organoids. Development, 144(21), 3894–3906
Beccari, L., et al. (2018). Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids. Nature, 562(7726), 272–276
Harrison, S. E., et al. (2017). Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro. Science, 356(6334), eaal1810
Rivron, N. C., et al. (2018). Blastocyst-like structures generated solely from stem cells. Nature, 557(7703), 106–111
Sozen, B., et al. (2018). Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures. Nature Cell Biology, 20(8), 979–989
Zhang, S., et al. (2019). Implantation initiation of self-assembled embryo-like structures generated using three types of mouse blastocyst-derived stem cells. Nature Communications, 10(1), 496
Vrij, E. J., et al. (2019). Chemically-defined induction of a primitive endoderm and epiblast-like niche supports post-implantation progression from blastoids. bioRxiv 510396
Frias-Aldeguer, J., et al. (2020). Embryonic signals perpetuate polar-like trophoblast stem cells and pattern the blastocyst axis. bioRxiv 510362
Xu, P. F., et al. (2021). Construction of a mammalian embryo model from stem cells organized by a morphogen signalling centre. Nature Communications, 12(1), 3277
Veenvliet, J. V., et al. (2020). Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites. Science, 370(6522), eaba4937
Rossi, G., et al. (2021). Capturing Cardiogenesis in Gastruloids. Cell Stem Cell, 28(2), 230–240e6
Bérenger-Currias, N. M. L. P., et al. (2022). A gastruloid model of the interaction between embryonic and extra-embryonic cell types. Journal of Tissue Engineering, 13, 20417314221103042
van den Brink, S. C., et al. (2020). Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature, 582(7812), 405–409
Zhao, C., et al. (2021). Reprogrammed blastoids contain amnion-like cells but not trophectoderm. bioRxiv
Yu, L., et al. (2021). Blastocyst-like structures generated from human pluripotent stem cells. Nature, 591(7851), 620–626
Yanagida, A., et al. (2021). Naive stem cell blastocyst model captures human embryo lineage segregation. Cell Stem Cell, 28(6), 1016–1022. e4
Mukhopadhyay, M. (2021). 3D in vitro models of human blastocysts. Nature Methods, 18(5), 445
Moris, N., et al. (2021). Biomedical and societal impacts of in vitro embryo models of mammalian development. Stem Cell Reports, 16(5), 1021–1030
Liu, X., et al. (2021). Modelling human blastocysts by reprogramming fibroblasts into iBlastoids. Nature, 591(7851), 627–632
Martyn, I., et al. (2018). Self-organization of a human organizer by combined Wnt and Nodal signalling. Nature, 558(7708), 132–135
Simunovic, M., et al. (2019). A 3D model of a human epiblast reveals BMP4-driven symmetry breaking. Nature Cell Biology, 21(7), 900–910
Warmflash, A., et al. (2014). A method to recapitulate early embryonic spatial patterning in human embryonic stem cells. Nature Methods, 11(8), 847–854
Moris, N., et al. (2020). An in vitro model of early anteroposterior organization during human development. Nature, 582(7812), 410–415
Shao, Y., et al. (2017). Self-organized amniogenesis by human pluripotent stem cells in a biomimetic implantation-like niche. Nature Materials, 16(4), 419–425
Xue, X., et al. (2018). Mechanics-guided embryonic patterning of neuroectoderm tissue from human pluripotent stem cells. Nature Materials, 17(7), 633–641
Zheng, Y., et al. (2019). Controlled modelling of human epiblast and amnion development using stem cells. Nature, 573(7774), 421–425
Budjan, C., et al. (2022). Paraxial mesoderm organoids model development of human somites. Elife, 11, e68925
Sanaki-Matsumiya, M., et al. (2022). Periodic formation of epithelial somites from human pluripotent stem cells. Nature Communications, 13(1), 2325
Olmsted, Z. T., & Paluh, J. L. (2021). Co-development of central and peripheral neurons with trunk mesendoderm in human elongating multi-lineage organized gastruloids. Nature Communications, 12(1), 3020
Libby, A. R. G., et al. (2021). Axial elongation of caudalized human organoids mimics aspects of neural tube development. Development, 148(12), dev198275
Turner, D. A., et al. (2016). Organoids and the genetically encoded self-assembly of embryonic stem cells. Bioessays, 38(2), 181–191
Simunovic, M., & Brivanlou, A. H. (2017). Embryoids, organoids and gastruloids: new approaches to understanding embryogenesis. Development, 144(6), 976–985
Shahbazi, M. N., & Zernicka-Goetz, M. (2018). Deconstructing and reconstructing the mouse and human early embryo. Nature Cell Biology, 20(8), 878–887
Taniguchi, K., et al. (2019). Opening the black box: Stem cell-based modeling of human post-implantation development. Journal Of Cell Biology, 218(2), 410–421
Zylicz, J. J. (2020). Defined Stem Cell Culture Conditions to Model Mouse Blastocyst Development.Curr Protoc Stem Cell Biol52 (1), e105
World Health Organization (2022). Birth defects. https://www.who.int/news-room/fact-sheets/detail/birth-defects, (accessed 24/03/2022)
Wallingford, J. B. (2005). Neural tube closure and neural tube defects: Studies in animal models reveal known knowns and known unknowns. American Journal of Medical Genetics Part C: Seminars in Medical Genetics, 135 C(1), 59–68
Liu, A. (2020). Animal Models of Human Birth Defects (1st ed.). Springer Singapore
Lewis-Israeli, Y. R., et al. (2021). Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nature Communications, 12(1), 5142
Drakhlis, L., et al. (2021). Human heart-forming organoids recapitulate early heart and foregut development. Nature Biotechnology, 39(6), 737–746
Hofbauer, P., et al. (2021). Cardioids reveal self-organizing principles of human cardiogenesis. Cell, 184(12), 3299–3317e22
Shou, Y., et al. (2020). The Application of Brain Organoids: From Neuronal Development to Neurological Diseases. Front Cell Dev Biol, 8, 579659
Khoury, M. J. (1989). Epidemiology of birth defects. Epidemiologic Reviews, 11, 244–248
Fahed, A. C., et al. (2013). Genetics of Congenital Heart Disease. Circulation Research, 112(4), 707–720
Brutsaert, D. L. (2003). Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiological Reviews, 83(1), 59–115
Narmoneva, D. A., et al. (2004). Endothelial cells promote cardiac myocyte survival and spatial reorganization: implications for cardiac regeneration. Circulation, 110(8), 962–968
Kidokoro, H., et al. (2018). The heart tube forms and elongates through dynamic cell rearrangement coordinated with foregut extension. Development, 145(7), dev152488
Varner, V. D., & Taber, L. A. (2012). Not just inductive: a crucial mechanical role for the endoderm during heart tube assembly. Development, 139(9), 1680–1690
Olmsted, Z. T., & Paluh, J. L. (2022). A Combined Human Gastruloid Model of Cardiogenesis and Neurogenesis. bioRxiv, 2022.02.25.481991
Eckalbar, W. L., et al. (2012). Scoliosis and segmentation defects of the vertebrae. Wiley Interdisciplinary Reviews: Developmental Biology, 1(3), 401–423
Bejiqi, R., et al. (2015). Klippel - Feil Syndrome Associated with Congential Heart Disease Presentaion of Cases and a Review of the Curent Literature. Open access Macedonian journal of medical sciences, 3(1), 129–134
Simpson, J. M., et al. (1995). Congenital heart disease in spondylothoracic dysostosis: two familial cases. Journal Of Medical Genetics, 32(8), 633–635
Mortier, G. R., et al. (1996). Multiple vertebral segmentation defects: analysis of 26 new patients and review of the literature. American Journal Of Medical Genetics, 61(4), 310–319
Turnpenny, P. D., et al. (2007). Abnormal vertebral segmentation and the notch signaling pathway in man. Developmental Dynamics, 236(6), 1456–1474
Naiche, L. A., et al. (2011). FGF4 and FGF8 comprise the wavefront activity that controls somitogenesis. Proceedings of the National Academy of Sciences 108 (10), 4018–4023
Aulehla, A., et al. (2003). Wnt3a Plays a Major Role in the Segmentation Clock Controlling Somitogenesis. Developmental Cell, 4(3), 395–406
Maroto, M., et al. (2012).Somitogenesis. Development139 (14),2453–2456
Blencowe, H., et al. (2018). Estimates of global and regional prevalence of neural tube defects for 2015: a systematic analysis. Annals of the New York Academy of Sciences, 1414(1), 31–46
Ornoy, A., & Arnon, J. (1993). Clinical teratology. Western Journal Of Medicine, 159(3), 382–390
F.Gilbert, S., & Epel, D. (2009). Ecological Developmental Biology: Integrating Epigenetics, Medicine, And Evolution. 2nd Edition
European Medicines Agency (2020). ICH S5 (R3) guideline on reproductive toxicology: Detection of Toxicity to Reproduction for Human Pharmaceuticals. https://www.ema.europa.eu/en/ich-s5-r3-guideline-reproductive-toxicology-detection-toxicity-reproduction-human-pharmaceuticals, (accessed 24/03/2022)
Vargesson, N. (2015). Thalidomide-induced teratogenesis: history and mechanisms. Birth Defects Res C Embryo Today, 105(2), 140–156
Mellin, G. W., & Katzenstein, M. (1962). The saga of thalidomide. Neuropathy to embryopathy, with case reports of congenital anomalies. N Engl J Med 267, 1238-44 concl
Stummann, T. C., et al. (2007). Embryotoxicity hazard assessment of methylmercury and chromium using embryonic stem cells.Toxicology242 (1–3), 130 – 43.
Stummann, T. C., et al. (2008). Embryotoxicity hazard assessment of cadmium and arsenic compounds using embryonic stem cells.Toxicology252 (1–3), 118 – 22.
Genschow, E., et al. (2002). The ECVAM international validation study on in vitro embryotoxicity tests: results of the definitive phase and evaluation of prediction models. European Centre for the Validation of Alternative Methods. Alternatives To Laboratory Animals, 30(2), 151–176
Luz, A. L., & Tokar, E. J. (2018). Pluripotent Stem Cells in Developmental Toxicity Testing: A Review of Methodological Advances. Toxicological Sciences, 165(1), 31–39
Kugler, J., et al. (2017). Embryonic stem cells and the next generation of developmental toxicity testing. Expert Opinion On Drug Metabolism & Toxicology, 13(8), 833–841
Liu, W., et al. (2013). Stem cell models for drug discovery and toxicology studies. Journal Of Biochemical And Molecular Toxicology, 27(1), 17–27
Shinde, V., et al. (2015). Human Pluripotent Stem Cell Based Developmental Toxicity Assays for Chemical Safety Screening and Systems Biology Data Generation.J Vis Exp( 100), e52333
Meganathan, K., et al. (2012). Identification of thalidomide-specific transcriptomics and proteomics signatures during differentiation of human embryonic stem cells.PLoS One7 (8), e44228
Warkus, E. L. L., & Marikawa, Y. (2018). Fluoxetine Inhibits Canonical Wnt Signaling to Impair Embryoid Body Morphogenesis: Potential Teratogenic Mechanisms of a Commonly Used Antidepressant. Toxicological Sciences, 165(2), 372–388
Krug, A. K., et al. (2013). Human embryonic stem cell-derived test systems for developmental neurotoxicity: a transcriptomics approach. Archives Of Toxicology, 87(1), 123–143
Lauschke, K., et al. (2020). A novel human pluripotent stem cell-based assay to predict developmental toxicity. Archives Of Toxicology, 94(11), 3831–3846
Jaklin, M., et al. (2020). Focus on germ-layer markers: A human stem cell-based model for in vitro teratogenicity testing. Reproductive Toxicology, 98, 286–298
Lau, C. G., & Marikawa, Y. (2014). Morphology-based mammalian stem cell tests reveal potential developmental toxicity of donepezil. Molecular Reproduction And Development, 81(11), 994–1008
Marikawa, Y., et al. (2009). Aggregated P19 mouse embryonal carcinoma cells as a simple in vitro model to study the molecular regulations of mesoderm formation and axial elongation morphogenesis. Genesis, 47(2), 93–106
Li, A. S., & Marikawa, Y. (2016). Adverse effect of valproic acid on an in vitro gastrulation model entails activation of retinoic acid signaling. Reproductive Toxicology, 66, 68–83
Marikawa, Y., et al. (2020). Exposure-based assessment of chemical teratogenicity using morphogenetic aggregates of human embryonic stem cells. Reproductive Toxicology, 91, 74–91
Mantziou, V., et al. (2021). In vitro teratogenicity testing using a 3D, embryo-like gastruloid system. Reproductive Toxicology, 105, 72–90
Cerrizuela, S., et al. (2020). The role of teratogens in neural crest development. Birth Defects Res, 112(8), 584–632
Girgin, M. U., et al. (2021). Gastruloids generated without exogenous Wnt activation develop anterior neural tissues. Stem Cell Reports, 16(5), 1143–1155
Hiramatsu, R., et al. (2013). External mechanical cues trigger the establishment of the anterior-posterior axis in early mouse embryos. Developmental Cell, 27(2), 131–144
Cha, J., & Dey, S. K. (2014). Cadence of procreation: orchestrating embryo-uterine interactions. Seminars In Cell & Developmental Biology, 34, 56–64
Wang, H., & Dey, S. K. (2006). Roadmap to embryo implantation: clues from mouse models. Nature Reviews Genetics, 7(3), 185–199
Fouladi-Nashta, A. A., et al. (2005). Characterization of the uterine phenotype during the peri-implantation period for LIF-null, MF1 strain mice. Development Biology, 281(1), 1–21
Rosario, G. X., et al. (2014). The LIF-mediated molecular signature regulating murine embryo implantation. Biology Of Reproduction, 91(3), 66
Stewart, C. L., et al. (1992). Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature, 359(6390), 76–79
Goolam, M., et al. (2020). The transcriptional repressor Blimp1/PRDM1 regulates the maternal decidual response in mice. Nature Communications, 11(1), 2782
Osafune, K., et al. (2008). Marked differences in differentiation propensity among human embryonic stem cell lines. Nature Biotechnology, 26(3), 313–315
Siller, R., et al. (2016). Development of a rapid screen for the endodermal differentiation potential of human pluripotent stem cell lines. Scientific Reports, 6, 37178
Kim, K., et al. (2011). Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nature Biotechnology, 29(12), 1117–1119
Quattrocelli, M., et al. (2011). Intrinsic cell memory reinforces myogenic commitment of pericyte-derived iPSCs. The Journal Of Pathology, 223(5), 593–603
Bar-Nur, O., et al. (2011). Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet beta cells. Cell Stem Cell, 9(1), 17–23
Funding
This work was supported by the South African National Research Foundation (M.G., Competitive Support for Unrated Researchers, A.A. Postgraduate MSc Scholarship) and the University of Cape Town (M.G., Research Development Grant, S.R., Building Research Active Grant).
Author information
Authors and Affiliations
Contributions
MG conceived and designed the study. AA and SR performed the literature review and wrote the first draft of the manuscript. All authors contributed to the reading, editing and approval of the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Atoosa Amel and Simoné Rossouw contributed equally to this publication.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Amel, A., Rossouw, S. & Goolam, M. Gastruloids: A Novel System for Disease Modelling and Drug Testing. Stem Cell Rev and Rep 19, 104–113 (2023). https://doi.org/10.1007/s12015-022-10462-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10462-5