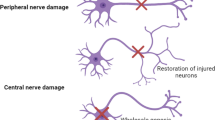
Abstract
Stem cell therapy holds great promise for the treatment of spinal cord injury (SCI), which can reverse neurodegeneration and promote tissue regeneration via its pluripotency and ability to secrete neurotrophic factors. Although various stem cell-based approaches have shown certain therapeutic effects when applied to the treatment of SCI, their clinical efficacies have been disappointing. Thus, it is an urgent need to further enhance the neurological benefits of stem cells through bioengineering strategies including genetic engineering. In this review, we summarize the progress of stem cell therapy for SCI and the prospect of genetically modified stem cells, focusing on the genome editing tools and functional molecules involved in SCI repair, trying to provide a deeper understanding of genetically modified stem cell therapy and more applicable clinical strategies for SCI repair.
Graphical abstract





Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this published article. The datasets analyzed during the study are available from the corresponding author on reasonable request.
Abbreviations
- SCI:
-
Spinal cord injury
- MSCs:
-
Mesenchymal stem cells
- HUCMSCs:
-
Human umbilical cord derived mesenchymal stem cells
- NSCs:
-
Neural stem cells
- IPSCs:
-
Induced pluripotent stem cells
- ESCs:
-
Embryonic stem cells
- BMSCs:
-
Bone marrow stromal cells
- HSPCs:
-
Hematopoietic stem and progenitor cells
- VEGF:
-
Vascular endothelial growth factor
- NGF:
-
Nerve growth factor
- GDNF:
-
Glial cell derived neurotrophic factor
- BDNF:
-
Brain-derived neurotrophic factor
- BFGF:
-
Basic fibroblast growth factor
- NT-3:
-
Neurotrophin-3
- NT-4/5:
-
Neurotrophin-4/5
- LIF:
-
Leukemia inhibitory factor
- SSN:
-
Site-specific nucleases
- HR:
-
Homologous recombination
- DSBs:
-
Double-strand breaks
- NHEJ:
-
Non-homologous end joining
- ZFNs:
-
Zinc finger nucleases
- TALENs:
-
Transcription activator-like effector nucleases
- CRISPR:
-
Clustered regularly interspaced short palindromic repeats
- IVT mRNA:
-
In vitro-transcribed mRNA
- CNS:
-
Central nervous system
- LTR:
-
Long terminal repeats
- LV:
-
Lentivirus
- Ngb:
-
Neuroglobin
- ROS:
-
Reactive oxygen species
- AAV:
-
Adeno-associated virus
- ITRs:
-
Inverted terminal repeats
- RNP:
-
Ribonucleoprotein
- DOTMA:
-
N - [1-(2,3-dioleyloxy) propyl]-N,N,N-trimethylammonium chloride
- DOTAP:
-
1,2-dioleoyl-3-trimethylammoniumpropane
- CHO:
-
Cholesterol
- DOPE:
-
Diolelphophatidylethanolamine
- DOPC:
-
Dioleylphosphatidylcholine
- LNPs:
-
Lipid nanoparticles
- PEG:
-
Polyethylene glycol
- DOGSDSO:
-
1′,2′ dioleoyl-sn-glycero-3′-succinyl-2-hydroxyethyl disulfide ornithine conjugate
- PEI:
-
Polyethylenimine
- CPPs:
-
Cell-penetrating peptides
- NAs:
-
Neural aggregates
- CST:
-
Corticospinal tract
References
Anjum, A., Yazid, M. D., Fauzi Daud, M., Idris, J., Ng, A. M. H., Selvi Naicker, A., et al. (2020). Spinal cord injury: Pathophysiology, multimolecular interactions, and underlying recovery mechanisms. International Journal of Molecular Sciences, 21(20), 7533.
(2014). Spinal cord injury facts and figures at a glance. The Journal of Spinal Cord Medicine, 37(1), 117–118.
Ackery, A., Tator, C., & Krassioukov, A. (2004). A global perspective on spinal cord injury epidemiology. Journal of Neurotrauma, 21(10), 1355–1370.
Silva, N. A., Sousa, N., Reis, R. L., & Salgado, A. J. (2014). From basics to clinical: A comprehensive review on spinal cord injury. Progress in Neurobiology, 114, 25–57.
Yip, P. K., & Malaspina, A. (2012). Spinal cord trauma and the molecular point of no return. Molecular Neurodegeneration, 7, 6.
Ulndreaj, A., Chio, J. C., Ahuja, C. S., & Fehlings, M. G. (2016). Modulating the immune response in spinal cord injury. Expert Review of Neurotherapeutics, 16(10), 1127–1129.
Schwab, M. E., & Strittmatter, S. M. (2014). Nogo limits neural plasticity and recovery from injury. Current Opinion in Neurobiology, 27, 53–60.
Bartlett, R. D., Burley, S., Ip, M., Phillips, J. B., & Choi, D. (2020). Cell therapies for spinal cord injury: Trends and challenges of current clinical trials. Neurosurgery., 87(4), E456–EE72.
Tetzlaff, W., Okon, E. B., Karimi-Abdolrezaee, S., Hill, C. E., Sparling, J. S., Plemel, J. R., et al. (2011). A systematic review of cellular transplantation therapies for spinal cord injury. Journal of Neurotrauma, 28(8), 1611–1682.
Assinck, P., Duncan, G. J., Hilton, B. J., Plemel, J. R., & Tetzlaff, W. (2017). Cell transplantation therapy for spinal cord injury. Nature Neuroscience, 20(5), 637–647.
Teng, Y. D., Lavik, E. B., Qu, X., Park, K. I., Ourednik, J., Zurakowski, D., et al. (2002). Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proceedings of the National Academy of Sciences of the United States of America, 99(5), 3024–3029.
Franz, S., Weidner, N., & Blesch, A. (2012). Gene therapy approaches to enhancing plasticity and regeneration after spinal cord injury. Experimental Neurology, 235(1), 62–69.
Wyse, R. D., Dunbar, G. L., & Rossignol, J. (2014). Use of genetically modified mesenchymal stem cells to treat neurodegenerative diseases. International Journal of Molecular Sciences, 15(2), 1719–1745.
Lee, H. J., Lim, I. J., Park, S. W., Kim, Y. B., Ko, Y., & Kim, S. U. (2012). Human neural stem cells genetically modified to express human nerve growth factor (NGF) gene restore cognition in the mouse with ibotenic acid-induced cognitive dysfunction. Cell Transplantation, 21(11), 2487–2496.
Park, D., Lee, H. J., Joo, S. S., Bae, D. K., Yang, G., Yang, Y. H., et al. (2012). Human neural stem cells over-expressing choline acetyltransferase restore cognition in rat model of cognitive dysfunction. Experimental Neurology, 234(2), 521–526.
Salehi, M. S., Safari, A., Pandamooz, S., Jurek, B., Hooshmandi, E., Owjfard, M., et al. (2021). The beneficial potential of genetically modified stem cells in the treatment of stroke: A review. Stem Cell Reviews and Reports, 18(2), 412–440.
Cofano, F., Boido, M., Monticelli, M., Zenga, F., Ducati, A., Vercelli, A., et al. (2019). Mesenchymal stem cells for spinal cord injury: Current options, limitations, and future of cell therapy. International Journal of Molecular Sciences, 20(11), 2698.
Thomson, J. A., Itskovitz-Eldor, J., Shapiro, S. S., Waknitz, M. A., Swiergiel, J. J., Marshall, V. S., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science., 282(5391), 1145–1147.
Shamblott, M. J., Axelman, J., Wang, S., Bugg, E. M., Littlefield, J. W., Donovan, P. J., et al. (1998). Derivation of pluripotent stem cells from cultured human primordial germ cells. Proceedings of the National Academy of Sciences of the United States of America, 95(23), 13726–13731.
Jin, M. C., Medress, Z. A., Azad, T. D., Doulames, V. M., & Veeravagu, A. (2019). Stem cell therapies for acute spinal cord injury in humans: A review. Neurosurgical Focus, 46(3), E10.
Gerrard, L., Rodgers, L., & Cui, W. (2005). Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells, 23(9), 1234–1241.
Keirstead, H. S., Nistor, G., Bernal, G., Totoiu, M., Cloutier, F., Sharp, K., et al. (2005). Human embryonic stem cell-derived oligodendrocyte progenitor cell transplants remyelinate and restore locomotion after spinal cord injury. The Journal of Neuroscience, 25(19), 4694–4705.
US National Library of Medicine. 2018 [Available from: https://clinicaltrials.gov/ct2/show/study/NCT02302157. Accessed 20 Jul 2021.]
White, S. V., Czisch, C. E., Han, M. H., Plant, C. D., Harvey, A. R., & Plant, G. W. (2016). Intravenous transplantation of mesenchymal progenitors distribute solely to the lungs and improve outcomes in cervical spinal cord injury. Stem Cells, 34(7), 1812–1825.
Chopp, M., Zhang, X. H., Li, Y., Wang, L., Chen, J., Lu, D., et al. (2000). Spinal cord injury in rat: Treatment with bone marrow stromal cell transplantation. Neuroreport., 11(13), 3001–3005.
Reynolds, B. A., & Weiss, S. (1992). Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science., 255(5052), 1707–1710.
Rosenzweig, E. S., Brock, J. H., Lu, P., Kumamaru, H., Salegio, E. A., Kadoya, K., et al. (2018). Restorative effects of human neural stem cell grafts on the primate spinal cord. Nature Medicine, 24(4), 484–490.
Mandai, M., Watanabe, A., Kurimoto, Y., Hirami, Y., Morinaga, C., Daimon, T., et al. (2017). Autologous induced stem-cell-derived retinal cells for macular degeneration. The New England Journal of Medicine, 376(11), 1038–1046.
Kawabata, S., Takano, M., Numasawa-Kuroiwa, Y., Itakura, G., Kobayashi, Y., Nishiyama, Y., et al. (2016). Grafted human iPS cell-derived oligodendrocyte precursor cells contribute to robust Remyelination of demyelinated axons after spinal cord injury. Stem Cell Reports, 6(1), 1–8.
Sieber-Blum, M. (2010). Epidermal neural crest stem cells and their use in mouse models of spinal cord injury. Brain Research Bulletin, 83(5), 189–193.
Pandamooz, S., Salehi, M. S., Nabiuni, M., Dargahi, L., & Pourghasem, M. (2016). Evaluation of epidermal neural crest stem cells in Organotypic spinal cord slice culture platform. Folia Biologica (Praha), 62(6), 263–267.
Kim, Y., Jo, S. H., Kim, W. H., & Kweon, O. K. (2015). Antioxidant and anti-inflammatory effects of intravenously injected adipose derived mesenchymal stem cells in dogs with acute spinal cord injury. Stem Cell Research & Therapy, 6, 229.
Chua, S. J., Bielecki, R., Yamanaka, N., Fehlings, M. G., Rogers, I. M., & Casper, R. F. (2010). The effect of umbilical cord blood cells on outcomes after experimental traumatic spinal cord injury. Spine (Phila Pa 1976), 35(16), 1520–1526.
Kao, C. H., Chen, S. H., Chio, C. C., & Lin, M. T. (2008). Human umbilical cord blood-derived CD34+ cells may attenuate spinal cord injury by stimulating vascular endothelial and neurotrophic factors. Shock., 29(1), 49–55.
Uchida, K., Nakajima, H., Guerrero, A. R., Johnson, W. E., Masri, W. E., & Baba, H. (2014). Gene therapy strategies for the treatment of spinal cord injury. Therapeutic Delivery, 5(5), 591–607.
Sykova, E., Jendelova, P., Urdzikova, L., Lesny, P., & Hejcl, A. (2006). Bone marrow stem cells and polymer hydrogels--two strategies for spinal cord injury repair. Cellular and Molecular Neurobiology, 26(7–8), 1113–1129.
Kadoya, K., Lu, P., Nguyen, K., Lee-Kubli, C., Kumamaru, H., Yao, L., et al. (2016). Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nature Medicine, 22(5), 479–487.
Pandamooz, S., Salehi, M. S., Zibaii, M. I., Ahmadiani, A., Nabiuni, M., & Dargahi, L. (2018). Epidermal neural crest stem cell-derived glia enhance neurotrophic elements in an ex vivo model of spinal cord injury. Journal of Cellular Biochemistry, 119(4), 3486–3496.
Vismara, I., Papa, S., Rossi, F., Forloni, G., & Veglianese, P. (2017). Current options for cell therapy in spinal cord injury. Trends in Molecular Medicine, 23(9), 831–849.
Teng, Y. D., Yu, D., Ropper, A. E., Li, J., Kabatas, S., Wakeman, D. R., et al. (2011). Functional multipotency of stem cells: A conceptual review of neurotrophic factor-based evidence and its role in translational research. Current Neuropharmacology, 9(4), 574–585.
Teng, Y. D. (2019). Functional multipotency of stem cells: Biological traits gleaned from neural progeny studies. Seminars in Cell & Developmental Biology, 95, 74–83.
Widenfalk, J., Lundstromer, K., Jubran, M., Brene, S., & Olson, L. (2001). Neurotrophic factors and receptors in the immature and adult spinal cord after mechanical injury or kainic acid. The Journal of Neuroscience, 21(10), 3457–3475.
Arvanian, V. L., & Mendell, L. M. (2001). Acute modulation of synaptic transmission to motoneurons by BDNF in the neonatal rat spinal cord. The European Journal of Neuroscience, 14(11), 1800–1808.
Ji, W. C., Zhang, X. W., & Qiu, Y. S. (2016). Selected suitable seed cell, scaffold and growth factor could maximize the repair effect using tissue engineering method in spinal cord injury. World Journal of Experimental Medicine, 6(3), 58–62.
Feng, S. Q., Kong, X. H., Liu, Y., Ban, D. X., Ning, G. Z., Chen, J. T., et al. (2009). Regeneration of spinal cord with cell and gene therapy. Orthopaedic Surgery, 1(2), 153–163.
Wang, J. M., Zeng, Y. S., Liu, R. Y., Huang, W. L., Xiong, Y., Wang, Y. H., et al. (2007). Recombinant adenovirus vector-mediated functional expression of neurotropin-3 receptor (TrkC) in neural stem cells. Experimental Neurology, 203(1), 123–127.
Mortazavi, M. M., Verma, K., Harmon, O. A., Griessenauer, C. J., Adeeb, N., Theodore, N., et al. (2015). The microanatomy of spinal cord injury: A review. Clinical Anatomy, 28(1), 27–36.
Liu, N. K., & Xu, X. M. (2012). Neuroprotection and its molecular mechanism following spinal cord injury. Neural Regeneration Research, 7(26), 2051–2062.
Guerrero, A. R., Uchida, K., Nakajima, H., Watanabe, S., Nakamura, M., Johnson, W. E., et al. (2012). Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. Journal of Neuroinflammation, 9, 40.
Leibinger, M., Zeitler, C., Gobrecht, P., Andreadaki, A., Gisselmann, G., & Fischer, D. (2021). Transneuronal delivery of hyper-interleukin-6 enables functional recovery after severe spinal cord injury in mice. Nature Communications, 12(1), 391.
Wang, L., Wei, F. X., Cen, J. S., Ping, S. N., Li, Z. Q., Chen, N. N., et al. (2014). Early administration of tumor necrosis factor-alpha antagonist promotes survival of transplanted neural stem cells and axon myelination after spinal cord injury in rats. Brain Research, 1575, 87–100.
Silver, J., & Miller, J. H. (2004). Regeneration beyond the glial scar. Nature Reviews. Neuroscience, 5(2), 146–156.
Rosenzweig, E. S., Salegio, E. A., Liang, J. J., Weber, J. L., Weinholtz, C. A., Brock, J. H., et al. (2019). Chondroitinase improves anatomical and functional outcomes after primate spinal cord injury. Nature Neuroscience, 22(8), 1269–1275.
Kanno, H., Pressman, Y., Moody, A., Berg, R., Muir, E. M., Rogers, J. H., et al. (2014). Combination of engineered Schwann cell grafts to secrete neurotrophin and chondroitinase promotes axonal regeneration and locomotion after spinal cord injury. The Journal of Neuroscience, 34(5), 1838–1855.
Griffin, J. M., Fackelmeier, B., Clemett, C. A., Fong, D. M., Mouravlev, A., Young, D., et al. (2020). Astrocyte-selective AAV-ADAMTS4 gene therapy combined with hindlimb rehabilitation promotes functional recovery after spinal cord injury. Experimental Neurology, 327, 113232.
Domeniconi, M., & Filbin, M. T. (2005). Overcoming inhibitors in myelin to promote axonal regeneration. Journal of the Neurological Sciences, 233(1–2), 43–47.
Wang, D., Liang, J., Zhang, J., Liu, S., & Sun, W. (2014). Mild hypothermia combined with a scaffold of NgR-silenced neural stem cells/Schwann cells to treat spinal cord injury. Neural Regeneration Research, 9(24), 2189–2196.
Zorner, B., & Schwab, M. E. (2010). Anti-Nogo on the go: From animal models to a clinical trial. Annals of the New York Academy of Sciences, 1198(Suppl 1), E22–E34.
Kucher, K., Johns, D., Maier, D., Abel, R., Badke, A., Baron, H., et al. (2018). First-in-man intrathecal application of neurite growth-promoting anti-Nogo-a antibodies in acute spinal cord injury. Neurorehabilitation and Neural Repair, 32(6–7), 578–589.
Wakeman, D. R., Redmond Jr., D. E., Dodiya, H. B., Sladek Jr., J. R., Leranth, C., Teng, Y. D., et al. (2014). Human neural stem cells survive long term in the midbrain of dopamine-depleted monkeys after GDNF overexpression and project neurites toward an appropriate target. Stem Cells Translational Medicine, 3(6), 692–701.
Dergham, P., Ellezam, B., Essagian, C., Avedissian, H., Lubell, W. D., & McKerracher, L. (2002). Rho signaling pathway targeted to promote spinal cord repair. The Journal of Neuroscience, 22(15), 6570–6577.
Liu, K., Lu, Y., Lee, J. K., Samara, R., Willenberg, R., Sears-Kraxberger, I., et al. (2010). PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nature Neuroscience, 13(9), 1075–1081.
Jin, D., Liu, Y., Sun, F., Wang, X., Liu, X., & He, Z. (2015). Restoration of skilled locomotion by sprouting corticospinal axons induced by co-deletion of PTEN and SOCS3. Nature Communications, 6, 8074.
Liu, Y., Wang, X., Li, W., Zhang, Q., Li, Y., Zhang, Z., et al. (2017). A sensitized IGF1 treatment restores corticospinal axon-dependent functions. Neuron., 95(4), 817–33 e4.
Kolevzon, A., Bush, L., Wang, A. T., Halpern, D., Frank, Y., Grodberg, D., et al. (2014). A pilot controlled trial of insulin-like growth factor-1 in children with Phelan-McDermid syndrome. Molecular Autism, 5(1), 54.
Kabu, S., Gao, Y., Kwon, B. K., & Labhasetwar, V. (2015). Drug delivery, cell-based therapies, and tissue engineering approaches for spinal cord injury. Journal of Controlled Release, 219, 141–154.
Sakamoto, K., Karelina, K., & Obrietan, K. (2011). CREB: A multifaceted regulator of neuronal plasticity and protection. Journal of Neurochemistry, 116(1), 1–9.
Bo, X., Wu, D., Yeh, J., & Zhang, Y. (2011). Gene therapy approaches for neuroprotection and axonal regeneration after spinal cord and spinal root injury. Current Gene Therapy, 11(2), 101–115.
Li, C., Li, X., Zhao, B., & Wang, C. (2020). Exosomes derived from miR-544-modified mesenchymal stem cells promote recovery after spinal cord injury. Archives of Physiology and Biochemistry, 126(4), 369–375.
Tebas, P., Stein, D., Tang, W. W., Frank, I., Wang, S. Q., Lee, G., et al. (2014). Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. The New England Journal of Medicine, 370(10), 901–910.
Hotta, A., & Yamanaka, S. (2015). From genomics to gene therapy: Induced pluripotent stem cells meet genome editing. Annual Review of Genetics, 49, 47–70.
Capecchi, M. R. (1989). Altering the genome by homologous recombination. Science., 244(4910), 1288–1292.
Zhang, H. X., Zhang, Y., & Yin, H. (2019). Genome editing with mRNA encoding ZFN, TALEN, and Cas9. Molecular Therapy, 27(4), 735–746.
Guo, J., Gaj, T., & Barbas 3rd., C. F. (2010). Directed evolution of an enhanced and highly efficient FokI cleavage domain for zinc finger nucleases. Journal of Molecular Biology, 400(1), 96–107.
Joung, J. K., & Sander, J. D. (2013). TALENs: A widely applicable technology for targeted genome editing. Nature Reviews. Molecular Cell Biology, 14(1), 49–55.
Mohanraju, P., Makarova, K. S., Zetsche, B., Zhang, F., Koonin, E. V., & van der Oost, J. (2016). Diverse evolutionary roots and mechanistic variations of the CRISPR-Cas systems. Science., 353(6299), aad5147.
Cohen, J. (2019). Prime editing promises to be a cut above CRISPR. Science., 366(6464), 406.
Moses, C., Hodgetts, S. I., Nugent, F., Ben-Ary, G., Park, K. K., Blancafort, P., et al. (2020). Transcriptional repression of PTEN in neural cells using CRISPR/dCas9 epigenetic editing. Scientific Reports, 10(1), 11393.
Klatt Shaw, D., Saraswathy, V. M., Zhou, L., McAdow, A. R., Burris, B., Butka, E., et al. (2021). Localized EMT reprograms glial progenitors to promote spinal cord repair. Developmental Cell, 56(5), 613–26 e7.
Yin, H., Kauffman, K. J., & Anderson, D. G. (2017). Delivery technologies for genome editing. Nature Reviews. Drug Discovery, 16(6), 387–399.
Xu, X., Wan, T., Xin, H., Li, D., Pan, H., Wu, J., et al. (2019). Delivery of CRISPR/Cas9 for therapeutic genome editing. The Journal of Gene Medicine, 21(7), e3107.
Leonhardt, C., Schwake, G., Stogbauer, T. R., Rappl, S., Kuhr, J. T., Ligon, T. S., et al. (2014). Single-cell mRNA transfection studies: Delivery, kinetics and statistics by numbers. Nanomedicine., 10(4), 679–688.
Liu, J., Gaj, T., Yang, Y., Wang, N., Shui, S., Kim, S., et al. (2015). Efficient delivery of nuclease proteins for genome editing in human stem cells and primary cells. Nature Protocols, 10(11), 1842–1859.
Chew, W. L. (2018). Immunity to CRISPR Cas9 and Cas12a therapeutics. Wiley Interdisciplinary Reviews. Systems Biology and Medicine, 10(1), e1408.
Bevan, A. K., Duque, S., Foust, K. D., Morales, P. R., Braun, L., Schmelzer, L., et al. (2011). Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Molecular Therapy, 19(11), 1971–1980.
Zhang, Y., Zheng, Y., Zhang, Y. P., Shields, L. B., Hu, X., Yu, P., et al. (2010). Enhanced adenoviral gene delivery to motor and dorsal root ganglion neurons following injection into demyelinated peripheral nerves. Journal of Neuroscience Research, 88(11), 2374–2384.
Ruitenberg, M. J., Plant, G. W., Hamers, F. P., Wortel, J., Blits, B., Dijkhuizen, P. A., et al. (2003). Ex vivo adenoviral vector-mediated neurotrophin gene transfer to olfactory ensheathing glia: Effects on rubrospinal tract regeneration, lesion size, and functional recovery after implantation in the injured rat spinal cord. The Journal of Neuroscience, 23(18), 7045–7058.
Liu, Y., Himes, B. T., Moul, J., Huang, W., Chow, S. Y., Tessler, A., et al. (1997). Application of recombinant adenovirus for in vivo gene delivery to spinal cord. Brain Research, 768(1–2), 19–29.
Nakajima, H., Uchida, K., Yayama, T., Kobayashi, S., Guerrero, A. R., Furukawa, S., et al. (2010). Targeted retrograde gene delivery of brain-derived neurotrophic factor suppresses apoptosis of neurons and oligodendroglia after spinal cord injury in rats. Spine (Phila Pa 1976), 35(5), 497–504.
Uchida, K., Nakajima, H., Hirai, T., Yayama, T., Chen, K., Guerrero, A. R., et al. (2012). The retrograde delivery of adenovirus vector carrying the gene for brain-derived neurotrophic factor protects neurons and oligodendrocytes from apoptosis in the chronically compressed spinal cord of twy/twy mice. Spine (Phila Pa 1976), 37(26), 2125–2135.
Liu, Y., Himes, B. T., Tryon, B., Moul, J., Chow, S. Y., Jin, H., et al. (1998). Intraspinal grafting of fibroblasts genetically modified by recombinant adenoviruses. Neuroreport., 9(6), 1075–1079.
Robbins, P. D., & Ghivizzani, S. C. (1998). Viral vectors for gene therapy. Pharmacology & Therapeutics, 80(1), 35–47.
Murray, M., & Fischer, I. (2001). Transplantation and gene therapy: Combined approaches for repair of spinal cord injury. Neuroscientist., 7(1), 28–41.
Blessing, D., & Deglon, N. (2016). Adeno-associated virus and lentivirus vectors: A refined toolkit for the central nervous system. Current Opinion in Virology, 21, 61–66.
Tom, V. J., Sandrow-Feinberg, H. R., Miller, K., Domitrovich, C., Bouyer, J., Zhukareva, V., et al. (2013). Exogenous BDNF enhances the integration of chronically injured axons that regenerate through a peripheral nerve grafted into a chondroitinase-treated spinal cord injury site. Experimental Neurology, 239, 91–100.
Lin, W. P., Chen, X. W., Zhang, L. Q., Wu, C. Y., Huang, Z. D., & Lin, J. H. (2013). Effect of neuroglobin genetically modified bone marrow mesenchymal stem cells transplantation on spinal cord injury in rabbits. PLoS ONE, 8(5), e63444.
Bedbrook, C. N., Deverman, B. E., & Gradinaru, V. (2018). Viral strategies for targeting the central and peripheral nervous systems. Annual Review of Neuroscience, 41, 323–348.
Grosse, S., Penaud-Budloo, M., Herrmann, A. K., Borner, K., Fakhiri, J., Laketa, V., et al. (2017). Relevance of assembly-activating protein for adeno-associated virus vector production and capsid protein stability in mammalian and insect cells. Journal of Virology, 91(20), e01198–17.
Rolling, F., & Samulski, R. J. (1995). AAV as a viral vector for human gene therapy. Generation of recombinant virus. Molecular Biotechnology, 3(1), 9–15.
Samulski, R. J., & Muzyczka, N. (2014). AAV-mediated gene therapy for research and therapeutic purposes. The Annual Review of Virology, 1(1), 427–451.
Kaeppel, C., Beattie, S. G., Fronza, R., van Logtenstein, R., Salmon, F., Schmidt, S., et al. (2013). A largely random AAV integration profile after LPLD gene therapy. Nature Medicine, 19(7), 889–891.
Blits, B., Oudega, M., Boer, G. J., Bartlett Bunge, M., & Verhaagen, J. (2003). Adeno-associated viral vector-mediated neurotrophin gene transfer in the injured adult rat spinal cord improves hind-limb function. Neuroscience., 118(1), 271–281.
Nassi, J. J., Cepko, C. L., Born, R. T., & Beier, K. T. (2015). Neuroanatomy goes viral! Frontiers in Neuroanatomy, 9, 80.
Hutson, T. H., Verhaagen, J., Yanez-Munoz, R. J., & Moon, L. D. (2012). Corticospinal tract transduction: A comparison of seven adeno-associated viral vector serotypes and a non-integrating lentiviral vector. Gene Therapy, 19(1), 49–60.
Buchholz, C. J., Friedel, T., & Buning, H. (2015). Surface-engineered viral vectors for selective and cell type-specific gene delivery. Trends in Biotechnology, 33(12), 777–790.
Yin, H., Kanasty, R. L., Eltoukhy, A. A., Vegas, A. J., Dorkin, J. R., & Anderson, D. G. (2014). Non-viral vectors for gene-based therapy. Nature Reviews. Genetics, 15(8), 541–555.
Wells, D. J. (2004). Gene therapy progress and prospects: Electroporation and other physical methods. Gene Therapy, 11(18), 1363–1369.
De Vry, J., Martinez-Martinez, P., Losen, M., Temel, Y., Steckler, T., Steinbusch, H. W., et al. (2010). In vivo electroporation of the central nervous system: A non-viral approach for targeted gene delivery. Progress in Neurobiology, 92(3), 227–244.
De Ravin, S. S., Reik, A., Liu, P. Q., Li, L., Wu, X., Su, L., et al. (2016). Targeted gene addition in human CD34(+) hematopoietic cells for correction of X-linked chronic granulomatous disease. Nature Biotechnology, 34(4), 424–429.
Mock, U., Machowicz, R., Hauber, I., Horn, S., Abramowski, P., Berdien, B., et al. (2015). mRNA transfection of a novel TAL effector nuclease (TALEN) facilitates efficient knockout of HIV co-receptor CCR5. Nucleic Acids Research, 43(11), 5560–5571.
Shimamura, M., Sato, N., Taniyama, Y., Kurinami, H., Tanaka, H., Takami, T., et al. (2005). Gene transfer into adult rat spinal cord using naked plasmid DNA and ultrasound microbubbles. The Journal of Gene Medicine, 7(11), 1468–1474.
Ando, T., Sato, S., Toyooka, T., Kobayashi, H., Nawashiro, H., Ashida, H., et al. (2012). Photomechanical wave-driven delivery of siRNAs targeting intermediate filament proteins promotes functional recovery after spinal cord injury in rats. PLoS ONE, 7(12), e51744.
Guan, S., & Rosenecker, J. (2017). Nanotechnologies in delivery of mRNA therapeutics using nonviral vector-based delivery systems. Gene Therapy, 24(3), 133–143.
Felgner, P. L., Gadek, T. R., Holm, M., Roman, R., Chan, H. W., Wenz, M., et al. (1987). Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proceedings of the National Academy of Sciences of the United States of America, 84(21), 7413–7417.
Kauffman, K. J., Dorkin, J. R., Yang, J. H., Heartlein, M. W., DeRosa, F., Mir, F. F., et al. (2015). Optimization of lipid nanoparticle formulations for mRNA delivery in vivo with fractional factorial and definitive screening designs. Nano Letters, 15(11), 7300–7306.
Immordino, M. L., Dosio, F., & Cattel, L. (2006). Stealth liposomes: Review of the basic science, rationale, and clinical applications, existing and potential. International Journal of Nanomedicine, 1(3), 297–315.
Ajmani, P. S., Tang, F., Krishnaswami, S., Meyer, E. M., Sumners, C., & Hughes, J. A. (1999). Enhanced transgene expression in rat brain cell cultures with a disulfide-containing cationic lipid. Neuroscience Letters, 277(3), 141–144.
Ohki, E. C., Tilkins, M. L., Ciccarone, V. C., & Price, P. J. (2001). Improving the transfection efficiency of post-mitotic neurons. Journal of Neuroscience Methods, 112(2), 95–99.
Obata, Y., Ciofani, G., Raffa, V., Cuschieri, A., Menciassi, A., Dario, P., et al. (2010). Evaluation of cationic liposomes composed of an amino acid-based lipid for neuronal transfection. Nanomedicine., 6(1), 70–77.
Samal, S. K., Dash, M., Van Vlierberghe, S., Kaplan, D. L., Chiellini, E., van Blitterswijk, C., et al. (2012). Cationic polymers and their therapeutic potential. Chemical Society Reviews, 41(21), 7147–7194.
Bus, T., Traeger, A., & Schubert, U. S. (2018). The great escape: How cationic polyplexes overcome the endosomal barrier. Journal of Materials Chemistry B, 6(43), 6904–6918.
Zhang, C., Yadava, P., & Hughes, J. (2004). Polyethylenimine strategies for plasmid delivery to brain-derived cells. Methods., 33(2), 144–150.
Kwon, E. J., Bergen, J. M., Park, I. K., & Pun, S. H. (2008). Peptide-modified vectors for nucleic acid delivery to neurons. Journal of Controlled Release, 132(3), 230–235.
Edgar, J. M., Robinson, M., & Willerth, S. M. (2017). Fibrin hydrogels induce mixed dorsal/ventral spinal neuron identities during differentiation of human induced pluripotent stem cells. Acta Biomaterialia, 51, 237–245.
Whittlesey, K. J., & Shea, L. D. (2006). Nerve growth factor expression by PLG-mediated lipofection. Biomaterials., 27(11), 2477–2486.
Kotterman, M. A., Chalberg, T. W., & Schaffer, D. V. (2015). Viral vectors for gene therapy: Translational and clinical outlook. Annual Review of Biomedical Engineering, 17, 63–89.
Yao, L., Yao, S., Daly, W., Hendry, W., Windebank, A., & Pandit, A. (2012). Non-viral gene therapy for spinal cord regeneration. Drug Discovery Today, 17(17–18), 998–1005.
Burnside, E. R., De Winter, F., Didangelos, A., James, N. D., Andreica, E. C., Layard-Horsfall, H., et al. (2018). Immune-evasive gene switch enables regulated delivery of chondroitinase after spinal cord injury. Brain., 141(8), 2362–2381.
Wang, L. J., Zhang, R. P., & Li, J. D. (2014). Transplantation of neurotrophin-3-expressing bone mesenchymal stem cells improves recovery in a rat model of spinal cord injury. Acta Neurochirurgica, 156(7), 1409–1418.
Sasaki, M., Radtke, C., Tan, A. M., Zhao, P., Hamada, H., Houkin, K., et al. (2009). BDNF-hypersecreting human mesenchymal stem cells promote functional recovery, axonal sprouting, and protection of corticospinal neurons after spinal cord injury. The Journal of Neuroscience, 29(47), 14932–14941.
Galieva, L. R., Mukhamedshina, Y. O., Akhmetzyanova, E. R., Gilazieva, Z. E., Arkhipova, S. S., Garanina, E. E., et al. (2018). Influence of genetically modified human umbilical cord blood mononuclear cells on the expression of Schwann cell molecular determinants in spinal cord injury. Stem Cells International, 2018, 4695275.
Muir, E., De Winter, F., Verhaagen, J., & Fawcett, J. (2019). Recent advances in the therapeutic uses of chondroitinase ABC. Experimental Neurology, 321, 113032.
Li, X., Peng, Z., Long, L., Tuo, Y., Wang, L., Zhao, X., et al. (2020). Wnt4-modified NSC transplantation promotes functional recovery after spinal cord injury. The FASEB Journal, 34(1), 82–94.
Song, J. L., Zheng, W., Chen, W., Qian, Y., Ouyang, Y. M., & Fan, C. Y. (2017). Lentivirus-mediated microRNA-124 gene-modified bone marrow mesenchymal stem cell transplantation promotes the repair of spinal cord injury in rats. Experimental & Molecular Medicine, 49(5), e332.
Huang, F., Gao, T., Wang, W., Wang, L., Xie, Y., Tai, C., et al. (2021). Engineered basic fibroblast growth factor-overexpressing human umbilical cord-derived mesenchymal stem cells improve the proliferation and neuronal differentiation of endogenous neural stem cells and functional recovery of spinal cord injury by activating the PI3K-Akt-GSK-3beta signaling pathway. Stem Cell Research & Therapy, 12(1), 468.
Li, X., & Dai, J. (2018). Bridging the gap with functional collagen scaffolds: Tuning endogenous neural stem cells for severe spinal cord injury repair. Biomaterials Science, 6(2), 265–271.
Liu, W., Xu, B., Xue, W., Yang, B., Fan, Y., Chen, B., et al. (2020). A functional scaffold to promote the migration and neuronal differentiation of neural stem/progenitor cells for spinal cord injury repair. Biomaterials., 243, 119941.
Li, G., Zhang, B., Sun, J. H., Shi, L. Y., Huang, M. Y., Huang, L. J., et al. (2021). An NT-3-releasing bioscaffold supports the formation of TrkC-modified neural stem cell-derived neural network tissue with efficacy in repairing spinal cord injury. Biomedical Materials, 6(11), 3766–3781.
Ropper, A. E., Thakor, D. K., Han, I., Yu, D., Zeng, X., Anderson, J. E., et al. (2017). Defining recovery neurobiology of injured spinal cord by synthetic matrix-assisted hMSC implantation. Proceedings of the National Academy of Sciences of the United States of America, 114(5), E820–E8E9.
Cheriyan, T., Ryan, D. J., Weinreb, J. H., Cheriyan, J., Paul, J. C., Lafage, V., et al. (2014). Spinal cord injury models: A review. Spinal Cord, 52(8), 588–595.
Pandamooz, S., Salehi, M. S., Zibaii, M. I., Safari, A., Nabiuni, M., Ahmadiani, A., et al. (2019). Modeling traumatic injury in organotypic spinal cord slice culture obtained from adult rat. Tissue & Cell, 56, 90–97.
Reier, P. J., Lane, M. A., Hall, E. D., Teng, Y. D., & Howland, D. R. (2012). Translational spinal cord injury research: Preclinical guidelines and challenges. Handbook of Clinical Neurology, 109, 411–433.
Acknowledgements
Thanks to the support of BioRender (https://app.biorender.com), all figures of the review were completed via the website.
Funding
This study was supported by the National Key Research and Development Program of China (2017YFA0104304), National Natural Science Foundation of China (NSFC) [81571213 and 82070459 (Bin Wang)], Key Project of Jiangsu Province (Grant No. BE2020765) (Bin Wang), Nanjing Medical Science and Technique Development Foundation (QRX17006, QRX17057, and ZKX20016) (Bin Wang), Jiangsu Provincial Plan for Mass Entrepreneurship and Innovation (2019) (Bin Wang), and Project of Modern Hospital Management and Development Institute, Nanjing University/Aid project of Nanjing Drum Tower Hospital Health, Education & Research Foundation (NDYG2020030) (Bin Wang).
Author information
Authors and Affiliations
Contributions
BW and PpS designed the study. YrF and YL performed the literature reviewing and writing. YrF wrote the first draft of the manuscript and all authors read, edited, and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Feng, Y., Li, Y., Shen, PP. et al. Gene-Modified Stem Cells for Spinal Cord Injury: a Promising Better Alternative Therapy. Stem Cell Rev and Rep 18, 2662–2682 (2022). https://doi.org/10.1007/s12015-022-10387-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10387-z