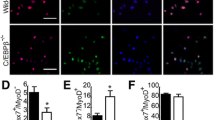
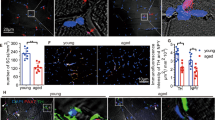
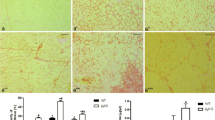
Abstract
Knockout (ko) mice for the β2 adrenoceptor (Adrβ2) have impaired skeletal muscle regeneration, suggesting that this receptor is important for muscle stem cell (satellite cell) function. Here, we investigated the role of Adrβ2 in the function of satellite cells from β2ko mice in the context of muscle regeneration, through in vivo and in vitro experiments. Immunohistochemical analysis showed a significant reduction in the number of self-renewed Pax7+ satellite cells, proliferating Pax7+/MyoD+ myogenic precursor cells, and regenerating eMHC+ myofibers in regenerating muscle of β2ko mice at 30, 3, and 10 days post-injury, respectively. Quiescent satellite cells were isolated by fluorescence-activated cell sorting, and cell cycle entry was assessed by EdU incorporation. The results demonstrated a lower number of proliferating Pax7+/EdU+ satellite cells from β2ko mice. There was an increase in the gene expression of the cell cycle inhibitor Cdkn1a and Notch pathway components and the activation of Notch signaling in proliferating myoblasts from β2ko mice. There was a decrease in the number of myogenin-positive nuclei in myofibers maintained in differentiation media, and a lower fusion index in differentiating myoblasts from β2ko mice. Furthermore, the gene expression of Wnt/β-catenin signaling components, the expression of nuclear β-catenin and the activation of Wnt/β-catenin signaling decreased in differentiating myoblasts from β2ko mice. These results indicate that Adrβ2 plays a crucial role in satellite cell self-renewal, as well as in myoblast proliferation and differentiation by regulating Notch and Wnt/β-catenin signaling, respectively.
Graphical abstract






Similar content being viewed by others
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
Change history
14 April 2022
A Correction to this paper has been published: https://doi.org/10.1007/s12015-022-10372-6
References
Mauro, A. (1961). Satellite cell of skeletal muscle fibers. The Journal of Biophysical and Biochemical Cytology, 9, 493–495.
Feige, P., Brun, C. E., Ritso, M., & Rudnicki, M. A. (2019). Orienting muscle stem cells for regeneration in homeostasis, aging, and disease. Cell Stem Cell, 23, 653–664.
Bentzinger, C. F., Wang, Y. X., Dumont, N. A., & Rudnicki, M. A. (2013). Cellular dynamics in the muscle satellite cell niche. EMBO Reports, 14, 1062–1072.
Dort, J., Fabre, P., Molina, T., & Dumont, N. A. (2019). Macrophages are key regulators of stem cells during skeletal muscle regeneration and diseases. Stem Cells International, 2019, 1–20.
Yin, H., Price, F., & Rudnicki, M. A. (2013). Satellite cells and the muscle stem cell niche. Physiological Reviews, 93, 23–67.
Giordani, L., Parisi, A., & Le Grand, F. (2018). Satellite cell self-renewal. Current Topics in Developmental Biology, 126, 177–203.
Rayagiri, S. S., Ranaldi, D., Raven, A., Mohamad Azhar, N. I. F., Lefebvre, O., Zammit, P. S., & Borycki, A.-G. (2018). Basal lamina remodeling at the skeletal muscle stem cell niche mediates stem cell self-renewal. Nature Communications, 9, 1075–1075.
Floss, T., Arnold, H. H., & Braun, T. (1997). A role for FGF-6 in skeletal muscle regeneration. Genes & Development, 11, 2040–2051.
Zanou, N., & Gailly, P. (2013). Skeletal muscle hypertrophy and regeneration: Interplay between the myogenic regulatory factors (MRFs) and insulin-like growth factors (IGFs) pathways. Cellular and Molecular Life Sciences, 70, 4117–4130.
Baghdadi, M. B., & Tajbakhsh, S. (2018). Regulation and phylogeny of skeletal muscle regeneration. Developmental Biology, 433, 200–209.
Cheung, T. H., & Rando, T. A. (2013). Molecular regulation of stem cell quiescence. Nature Reviews Molecular Cell Biology, 14, 329–340.
Bjornson, C. R. R., Cheung, T. H., Liu, L., Tripathi, P. V., Steeper, K. M., & Rando, T. A. (2012). Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells, 30, 232–242.
Tsivitse, S. (2010). Notch and Wnt signaling, physiological stimuli and postnatal Myogenesis. International Journal of Biological Sciences, 6, 268–281.
Silva, M. T., Wensing, L. A., Brum, P. C., Camara, N. O., & Miyabara, E. H. (2014). Impaired structural and functional regeneration of skeletal muscles from beta2-adrenoceptor knockout mice. Acta Physiologica (Oxford, England), 211, 617–633.
Silva, M. T., Nascimento, T. L., Pereira, M. G., Siqueira, A. S., Brum, P. C., Jaeger, R. G., & Miyabara, E. H. (2016). beta2-adrenoceptor is involved in connective tissue remodeling in regenerating muscles by decreasing the activity of MMP-9. Cell and Tissue Research, 365, 173–186.
Kim, Y. S., & Sainz, R. D. (1992). β-Adrenergic agonists and hypertrophy of skeletal muscles. Life Sciences, 50, 397–407.
Lynch, G. S., & Ryall, J. G. (2008). Role of β-adrenoceptor signaling in skeletal muscle: Implications for muscle wasting and disease. Physiological Reviews, 88, 729–767.
Koopman, R., Gehrig, S. M., Léger, B., Trieu, J., Walrand, S., Murphy, K. T., & Lynch, G. S. (2010). Cellular mechanisms underlying temporal changes in skeletal muscle protein synthesis and breakdown during chronic β-adrenoceptor stimulation in mice. Journal of Physiology, 588, 4811–4823.
Joassard, O. R., Amirouche, A., Gallot, Y. S., Desgeorges, M. M., Castells, J., Durieux, A. C., Berthon, P., & Freyssenet, D. G. (2013). Regulation of Akt-mTOR, ubiquitin-proteasome and autophagy-lysosome pathways in response to formoterol administration in rat skeletal muscle. International Journal of Biochemistry and Cell Biology, 45, 2444–2455.
Gonçalves, D. A., Silveira, W. A., Manfredi, L. H., Graça, F. A., Armani, A., Bertaggia, E., O’Neill, B. T., Lautherbach, N., Machado, J., Nogara, L., Pereira, M. G., Arcidiacono, D., Realdon, S., Kahn, C. R., Sandri, M., Kettelhut, I. C., & Navegantes, L. C. C. (2019). Insulin/IGF1 signalling mediates the effects of β 2 -adrenergic agonist on muscle proteostasis and growth. Journal of Cachexia, Sarcopenia and Muscle, 10, 455–475.
Hagg, A., Colgan, T. D., Thomson, R. E., Qian, H., Lynch, G. S., & Gregorevic, P. (2016). Using AAV vectors expressing the β2-adrenoceptor or associated Gα proteins to modulate skeletal muscle mass and muscle fibre size. Scientific Reports, 6, 23042–23042.
Chia, L. Y., Evans, B. A., Mukaida, S., Bengtsson, T., Hutchinson, D. S., & Sato, M. (2019). Adrenoceptor regulation of the mechanistic target of rapamycin in muscle and adipose tissue. British Journal of Pharmacology, 176, 2433–2448.
Conte, T. C., Silva, L. H., Silva, M. T., Hirabara, S. M., Oliveira, A. C., Curi, R., Moriscot, A. S., Aoki, M. S., & Miyabara, E. H. (2012). The beta2-adrenoceptor agonist formoterol improves structural and functional regenerative capacity of skeletal muscles from aged rat at the early stages of postinjury. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 67, 443–455.
Roberts, P., & Mcgeachie, J. K. (1992). The effects of clenbuterol on satellite cell activation and the regeneration of skeletal muscle: An autoradiographic and morphometric study of whole muscle transplants in mice. Journal of Anatomy, 180, 57–65.
Ryall, J. G., Schertzer, J. D., Alabakis, T. M., Gehrig, S. M., Plant, D. R., & Lynch, G. S. (2008). Intramuscular beta2-agonist administration enhances early regeneration and functional repair in rat skeletal muscle after myotoxic injury. Journal of Applied Physiology, 105, 165–172.
Chen, S.-J., Yue, J., Zhang, J.-X., Jiang, M., Hu, T.-Q., Leng, W.-D., Xiang, L., Li, X.-Y., Zhang, L., Zheng, F., Yuan, Y., Guo, L.-Y., Pan, Y.-M., Yan, Y.-W., Wang, J.-N., Chen, S.-Y., & Tang, J.-M. (2019). Continuous exposure of isoprenaline inhibits myoblast differentiation and fusion through PKA/ERK1/2-FOXO1 signaling pathway. Stem Cell Research & Therapy, 10, 70–70.
Chruscinski, A. J., Rohrer, D. K., Schauble, E., Desai, K. H., Bernstein, D., & Kobilka, B. K. (1999). Targeted disruption of the β2 adrenergic receptor gene. Journal of Biological Chemistry, 274, 16694–16700.
Gopinath, S. D., Webb, A. E., Brunet, A., & Rando, T. A. (2014). FOXO3 promotes quiescence in adult muscle stem cells during the process of self-renewal. Stem Cell Reports, 2, 414–426.
Cheung, T. H., Quach, N. L., Charville, G. W., Liu, L., Park, L., Edalati, A., Yoo, B., Hoang, P., & Rando, T. A. (2012). Maintenance of muscle stem-cell quiescence by microRNA-489. Nature, 482, 524–528.
Zammit, P. S., Partridge, T. A., & Yablonka-Reuveni, Z. (2006). The skeletal muscle satellite cell: The stem cell that came in from the cold. The Journal of Histochemistry and Cytochemistry, 54, 1177–1191.
Quach, N. L., & Rando, T. A. (2006). Focal adhesion kinase is essential for costamerogenesis in cultured skeletal muscle cells. Developmental Biology, 293, 38–52.
Wen, Y., Bi, P., Liu, W., Asakura, A., Keller, C., & Kuang, S. (2012). Constitutive notch activation upregulates Pax7 and promotes the self-renewal of skeletal muscle satellite cells. Molecular and Cellular Biology, 32, 2300–2311.
Fuziwara, C. S., & Kimura, E. T. (2014). High iodine blocks a notch/miR-19 loop activated by the BRAF(V600E) oncoprotein and restores the response to TGFbeta in thyroid follicular cells. Thyroid, 24, 453–462.
Ridgeway, A. G., Petropoulos, H., Wilton, S., & Skerjanc, I. S. (2000). Wnt signaling regulates the function of MyoD and Myogenin. Journal of Biological Chemistry, 275, 32398–32405.
Rudolf, A., Schirwis, E., Giordani, L., Parisi, A., Lepper, C., Taketo, M. M., & Le Grand, F. (2016). β-Catenin activation in muscle progenitor cells regulates tissue repair. Cell Reports, 15, 1277–1290.
Veeman, M. T., Slusarski, D. C., Kaykas, A., Louie, S. H., & Moon, R. T. (2003). Zebrafish prickle, a modulator of noncanonical Wnt/Fz signaling, regulates gastrulation movements. Current Biology, 13, 680–685.
Kanzleiter, T., Wilks, D., Preston, E., Ye, J., Frangioudakis, G., & Cooney, G. J. (2009). Regulation of the nuclear hormone receptor nur77 in muscle: Influence of exercise-activated pathways in vitro and obesity in vivo. Biochimica et Biophysica Acta - Molecular Basis of Disease, 1792, 777–782.
Maruoka, H., Yamazoe, R., Takahashi, R., Yatsuo, K., Ido, D., Fuchigami, Y., Hoshikawa, F., & Shimoke, K. (2020). Molecular mechanism of nur77 gene expression and downstream target genes in the early stage of forskolin-induced differentiation in PC12 cells. Scientific Reports, 10, 33–35.
Hawke, T. J., Meeson, A. P., Jiang, N., Graham, S., Hutcheson, K., Dimaio, J. M., & Garry, D. J. (2003). p21 is essential for normal myogenic progenitor cell function in regenerating skeletal muscle. American Journal of Physiology-Cell Physiology, 285, C1019–C1027.
Yosef, R., Pilpel, N., Papismadov, N., Gal, H., Ovadya, Y., Vadai, E., Miller, S., Porat, Z., Ben-Dor, S., & Krizhanovsky, V. (2017). p21 maintains senescent cell viability under persistent DNA damage response by restraining JNK and caspase signaling. The EMBO Journal, 36, 2280–2295.
Beitzel, F., Sillence, M. N., & Lynch, G. S. (2007). β-Adrenoceptor signaling in regenerating skeletal muscle after β-agonist administration. American Journal of Physiology. Endocrinology and Metabolism, 293, E932–E940.
Mourikis, P., Sambasivan, R., Castel, D., Rocheteau, P., Bizzarro, V., Tajbakhsh, S., & Philippos, M. (2012). A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells, 30, 243–252.
Luo, D., Renault, V. M., & Rando, T. A. (2005). The regulation of notch signaling in muscle stem cell activation and postnatal myogenesis. Seminars in Cell & Developmental Biology, 16, 612–622.
Alonso-Martin, S., Auradé, F., Mademtzoglou, D., Rochat, A., Zammit, P. S., & Relaix, F. (2018). SOXF factors regulate murine satellite cell self-renewal and function through inhibition of β-catenin activity. eLife, 7, 1–29.
Delday, M. I., & Maltin, C. A. (1997). Clenbuterol increases the expression of myogenin but not myoD in immobilized rat muscles. The American Journal of Physiology, 272, E941–E944.
Chen, H., Liu, D., Yang, Z., Sun, L., Deng, Q., Yang, S., Qian, L., Guo, L., Yu, M., Hu, M., Shi, M., & Guo, N. (2014). Adrenergic signaling promotes angiogenesis through endothelial cell-tumor cell crosstalk. Endocrine-Related Cancer, 21, 783–795.
Conboy, I. M., & Rando, T. A. (2002). The regulation of notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Developmental Cell, 3, 397–409.
Fukada, S. I., Yamaguchi, M., Kokubo, H., Ogawa, R., Uezumi, A., Yoneda, T., Matev, M. M., Motohashi, N., Ito, T., Zolkiewska, A., Johnson, R. L., Saga, Y., Miyagoe-Suzuki, Y., Tsujikawa, K., Takeda, S. I., & Yamamoto, H. (2011). Hesr1 and Hesr3 are essential to generate undifferentiated quiescent satellite cells and to maintain satellite cell numbers. Development, 138, 4609–4619.
Spencer, S. L., Cappell, S. D., Tsai, F.-C., Overton, K. W., Wang, C. L., & Meyer, T. (2013). The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell, 155, 369–383.
Yao, X., Yu, T., Zhao, C., Li, Y., Peng, Y., Xi, F., & Yang, G. (2017). Evodiamine promotes differentiation and inhibits proliferation of C2C12 muscle cells. International Journal of Molecular Medicine, 41, 1627–1634.
Biferi, M. G., Nicoletti, C., Falcone, G., Puggioni, E. M. R., Passaro, N., Mazzola, A., Pajalunga, D., Zaccagnini, G., Rizzuto, E., Auricchio, A., Zentilin, L., De Luca, G., Giacca, M., Martelli, F., Musio, A., Musarò, A., & Crescenzi, M. (2015). Proliferation of multiple cell types in the skeletal muscle tissue elicited by acute p21 suppression. Molecular Therapy, 23, 885–895.
Chinzei, N., Hayashi, S., Ueha, T., Fujishiro, T., Kanzaki, N., Hashimoto, S., Sakata, S., Kihara, S., Haneda, M., Sakai, Y., Kuroda, R., & Kurosaka, M. (2015). P21 deficiency delays regeneration of skeletal muscular tissue. PLoS One, 10, e0125765–e0125765.
Brack, A. S., Conboy, I. M., Conboy, M. J., Shen, J., & Rando, T. A. (2008). A temporal switch from notch to Wnt signaling in muscle stem cells is necessary for Normal adult Myogenesis. Cell Stem Cell, 2, 50–59.
Chen, A. E., Ginty, D. D., & Fan, C. M. (2005). Protein kinase a signalling via CREB controls myogenesis induced by Wnt proteins. Nature, 433, 317–322.
Stewart, R., Flechner, L., Montminy, M., & Berdeaux, R. (2011). CREB is activated by muscle injury and promotes muscle regeneration. PLoS One, 6, e24714.
Li, L., & Fan, C. M. (2017). A CREB-MPP7-AMOT regulatory Axis controls muscle stem cell expansion and self-renewal competence. Cell Reports, 21, 1253–1266.
Cornelison, D. D. W., Filla, M. S., Stanley, H. M., Rapraeger, A. C., Olwin, B. B. (2001). Syndecan-3 and Syndecan-4 Specifically Mark Skeletal Muscle Satellite Cells and Are Implicated in Satellite Cell Maintenance and Muscle Regeneration. Developmental Biology 239, 79–94
Cornelison, D. D. W., Wilcox-Adelman, S. A., Goetinck, P. F., Rauvala, H., Rapraeger, A. C., Olwin, B. B. (2004). Essential and separable roles for Syndecan-3 and Syndecan-4 in skeletal muscle development and regeneration. Genes Dev, 18(18): 2231–2236.
Acknowledgments
The authors are grateful to Chao Yun Irene Yan for providing cryosections of cephalic regions from HH19/20 chick embryos, Luiz C. Navegantes for stimulating discussions, and Anselmo S. Moriscot for allowing the use of the cryostat from his laboratory. This study was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Grant Nos. 14/23391-8 and 18/24946-4) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Fellowship/Grant No. 312142/2018-8). Tatiana E. Koike received a Ph.D. fellowship from FAPESP (Grant No. 17/09069-4).
Funding
This study was funded by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) (Grant Nos. 14/23391–8 and 18/24946–4) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (Fellowship/Grant No. 312142/2018–8). Tatiana E. Koike received a Ph.D. fellowship from FAPESP (Grant No. 17/09069–4).
Author information
Authors and Affiliations
Contributions
Conceptualization: Tatiana E. Koike, Elen H. Miyabara. Experimental performance and/or data analysis: Tatiana E. Koike, Cesar S. Fuziwara, Patricia C. Brum, Edna T. Kimura, Thomas. A. Rando, Elen H. Miyabara. Funding acquisition: Thomas. A. Rando, Elen H. Miyabara. Project administration: Tatiana E. Koike, Elen H. Miyabara. Supervision: Elen H. Miyabara. Manuscript preparation: Tatiana E. Koike, Elen H. Miyabara. Manuscript review: Tatiana E. Koike, Cesar S. Fuziwara, Patricia C. Brum, Edna T. Kimura, Thomas. A. Rando, Elen H. Miyabara.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
All applicable international, national, and institutional guidelines for the care and use of animals were followed. All animal experiments were approved by the Animal Research Ethics Committee of the Institute of Biomedical Sciences of the University of São Paulo under Protocol No. 106/2017.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Figure 5 was updated only in HTML, but not in the PDF version. In addition, the Supplementary Information for Tables S1 and S2 (or ESM 3) has to be replaced and updated.
Supplementary Information

ESM 1
(PNG 595 kb)
12015_2022_10334_MOESM1_ESM.tif
Fluorescence-activated cell sorting gating strategy of myogenic progenitors. Gates were defined based on unstained or single-stained samples. Cells were gated on side scatter (SSC) and forward scatter (FSC), and dead cells were excluded by DAPI dilactate staining. Representative FACS of CD45– CD31– Sca1– VCAM1+ cells. (TIF 9746 kb)

ESM 2
(PNG 632 kb)
A
Adrβ2 expression in FACS-purified activated satellite cells isolated from wild-type (WT) mice at 3 dpi and cultured for 24 h in wash medium. Blue: DAPI; White: Synd-4; Green: MyoD; Red: Adrβ2. Adrβ2 expression in early differentiating myoblasts (FACS-purified quiescent satellite cells cultured in wash medium for 24 h and in differentiation medium for 24 h) from WT mice. Blue: DAPI; White: Synd-4; Red: myogenin (MyoG); Green: Adrβ2. Bar: 50 μm. B Cryosections obtained from the cephalic regions from HH19/20 chick embryos and incubated with antibodies against MyoD and MyoG and Adrβ2 and DAPI are negative controls of these antibodies. Syndecan-4 is a marker of quiescent and activated satellite cells. Once activated, satellite cells maintain the expression of syndecan-4 for at least 96 h (Cornelison et al. 57). Therefore, satellite cells were stained with the antibody against syndecan-4 (Cornelison et al. 58). Myogenic stages—activation/proliferation or early differentiation— were identified using MyoD or MyoG, respectively. C Cross-sections of uninjured TA muscles from WT and β2ko mice stained with Pax7 (red), laminin (white), and DAPI (blue). Arrows indicate Pax7+ satellite cells. Bar: 20 μm. D Number of Pax7+ satellite cells per mm2 of uninjured TA muscles from WT and β2ko mice (four fields per animal were analyzed). Mean ± SEM of three different biological replicates per group. *p ≤ 0.05 vs. WT. E mRNA levels of Nur-77 in proliferating myoblasts (cultured in growth medium for 24 h) from WT and β2ko mice. Mean ± SEM of four biological replicates per group. *p ≤ 0.05 vs. WT. (TIF 9686 kb)
ESM 3
(DOC 90.0 kb)
Rights and permissions
About this article
Cite this article
Koike, T.E., Fuziwara, C.S., Brum, P.C. et al. Muscle Stem Cell Function Is Impaired in β2-Adrenoceptor Knockout Mice. Stem Cell Rev and Rep 18, 2431–2443 (2022). https://doi.org/10.1007/s12015-022-10334-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10334-y