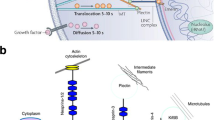
Abstract
Mesenchymal stem cells (MSCs) can sense and convert mechanical stimuli signals into a chemical response. Integrins are involved in the mechanotransduction from inside to outside and from outside to inside, and ultimately affect the fate of MSCs responding to different mechanical signals. Different integrins participate in different signaling pathways to regulate MSCs multi-differentiation. In this review, we summarize the latest advances in the effects of mechanical signals on the differentiation of MSCs, the importance of integrins in mechanotransduction, the relationship between integrin heterodimers and different mechanical signals, and the interaction among mechanical signals. We put forward our views on the prospect and challenges of developing mechanical biology in tissue engineering and regenerative medicine.
Graphical abstract




Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the cur- rent study are available from the corresponding author on reasonable request.
References
Horwitz, E. M., et al. (2005). Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy, 7(5), 393–395.
Guilak, F., & Mow, V. C. (2000). The mechanical environment of the chondrocyte: A biphasic finite element model of cell-matrix interactions in articular cartilage. Journal of Biomechanics, 33(12), 1663–1673.
Fletcher, D. A., & Mullins, R. D. (2010). Cell mechanics and the cytoskeleton. Nature, 463(7280), 485–492.
Jang, I. and K.A. Beningo, Integrins, CAFs and Mechanical Forces in the Progression of Cancer. Cancers (Basel), 2019. 11(5).
Gonzalez-Cruz, R. D., Fonseca, V. C., & Darling, E. M. (2012). Cellular mechanical properties reflect the differentiation potential of adipose-derived mesenchymal stem cells. Proc Natl Acad Sci U S A, 109(24), E1523–E1529.
Shattil, S. J., Kim, C., & Ginsberg, M. H. (2010). The final steps of integrin activation: The end game. Nature Reviews Molecular Cell Biology, 11(4), 288–300.
Ginsberg, M. H. (2014). Integrin activation. BMB Reports, 47(12), 655–659.
Li, J., & Springer, T. A. (2017). Integrin extension enables ultrasensitive regulation by cytoskeletal force. Proc Natl Acad Sci U S A, 114(18), 4685–4690.
Campbell, I.D. and M.J. Humphries, Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol, 2011. 3(3).
Xie, C., et al. (2010). Structure of an integrin with an alphaI domain, complement receptor type 4. EMBO Journal, 29(3), 666–679.
Shoemaker, B. A., Portman, J. J., & Wolynes, P. G. (2000). Speeding molecular recognition by using the folding funnel: The fly-casting mechanism. Proceedings of the National Academy of Sciences of the United States of America, 97(16), 8868–8873.
Horton, E. R., et al. (2016). The integrin adhesome network at a glance. Journal of Cell Science, 129(22), 4159–4163.
Klapholz, B., & Brown, N. H. (2017). Talin - the master of integrin adhesions. Journal of Cell Science, 130(15), 2435–2446.
Sun, Z., Costell, M., & Fassler, R. (2019). Integrin activation by talin, kindlin and mechanical forces. Nature Cell Biology, 21(1), 25–31.
Martino, M. M., et al. (2009). Controlling integrin specificity and stem cell differentiation in 2D and 3D environments through regulation of fibronectin domain stability. Biomaterials, 30(6), 1089–1097.
Cooper, J., & Giancotti, F. G. (2019). Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell, 35(3), 347–367.
Becchetti, A., Petroni, G., & Arcangeli, A. (2019). Ion Channel Conformations Regulate Integrin-Dependent Signaling. Trends in Cell Biology, 29(4), 298–307.
Pillozzi, S. and A. Becchetti, Ion channels in hematopoietic and mesenchymal stem cells. Stem Cells Int, 2012. 2012: p. 217910.
Xiao, E., et al. (2015). Brief reports: TRPM7 Senses mechanical stimulation inducing osteogenesis in human bone marrow mesenchymal stem cells. Stem Cells, 33(2), 615–621.
Arcangeli, A., & Becchetti, A. (2006). Complex functional interaction between integrin receptors and ion channels. Trends in Cell Biology, 16(12), 631–639.
Lawson, C.D. and K. Burridge, The on-off relationship of Rho and Rac during integrin-mediated adhesion and cell migration. Small GTPases, 2014. 5: p. e27958.
Ron, A., et al. (2017). Cell shape information is transduced through tension-independent mechanisms. Nature Communications, 8(1), 2145.
McIlhenny, S. E., et al. (2010). Linear shear conditioning improves vascular graft retention of adipose-derived stem cells by upregulation of the alpha5beta1 integrin. Tissue Engineering Part A, 16(1), 245–255.
Bächle, M., & Kohal, R. J. (2004). A systematic review of the influence of different titanium surfaces on proliferation, differentiation and protein synthesis of osteoblast-like MG63 cells. Clinical Oral Implants Research, 15(6), 683–692.
Reilly, G. C., & Engler, A. J. (2010). Intrinsic extracellular matrix properties regulate stem cell differentiation. Journal of Biomechanics, 43(1), 55–62.
Dejaeger, M., et al. (2017). Integrin-linked kinase regulates bone formation by controlling cytoskeletal organization and modulating BMP and Wnt signaling in osteoprogenitors. Journal of Bone and Mineral Research, 32(10), 2087–2102.
Park, H., et al. (2019). Integrin-linked kinase controls retinal angiogenesis and is linked to Wnt signaling and exudative vitreoretinopathy. Nature Communications, 10(1), 5243.
Sorcini, D., et al. (2017). Wnt/beta-catenin signaling induces integrin alpha4beta1 in T cells and promotes a progressive neuroinflammatory disease in mice. The Journal of Immunology, 199(9), 3031–3041.
Fouchard, J., et al. (2014). Three-dimensional cell body shape dictates the onset of traction force generation and growth of focal adhesions. Proc Natl Acad Sci U S A, 111(36), 13075–13080.
Wang, N., et al. (2001). Mechanical behavior in living cells consistent with the tensegrity model. Proceedings of the National Academy of Sciences, 98(14), 7765–7770.
Lavenus, S., et al. (2010). Cell interaction with nanopatterned surface of implants. Nanomedicine (London, England), 5(6), 937–947.
Lee, J., et al. (2015). Geometric guidance of integrin mediated traction stress during stem cell differentiation. Biomaterials, 69, 174–183.
Kilian, K. A., et al. (2010). Geometric cues for directing the differentiation of mesenchymal stem cells. Proc Natl Acad Sci U S A, 107(11), 4872–4877.
Mathieu, P. S., & Loboa, E. G. (2012). Cytoskeletal and focal adhesion influences on mesenchymal stem cell shape, mechanical properties, and differentiation down osteogenic, adipogenic, and chondrogenic pathways. Tissue Engineering. Part B, Reviews, 18(6), 436–444.
Yim, E. K., Pang, S. W., & Leong, K. W. (2007). Synthetic nanostructures inducing differentiation of human mesenchymal stem cells into neuronal lineage. Experimental Cell Research, 313(9), 1820–1829.
Lin, C. H., et al. (2018). Stiffness modification of photopolymerizable gelatin-methacrylate hydrogels influences endothelial differentiation of human mesenchymal stem cells. Journal of Tissue Engineering and Regenerative Medicine, 12(10), 2099–2111.
Guilak, F., et al. (2009). Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem Cell, 5(1), 17–26.
Nguyen, A.T., S.R. Sathe, and E.K. Yim, From nano to micro: topographical scale and its impact on cell adhesion, morphology and contact guidance. J Phys Condens Matter, 2016. 28(18): p. 183001.
Parandakh, A., et al. (2019). Substrate topography interacts with substrate stiffness and culture time to regulate mechanical properties and smooth muscle differentiation of mesenchymal stem cells. Colloids and Surfaces. B, Biointerfaces, 173, 194–201.
Hou, Y., et al., Surface roughness gradients reveal topography-specific mechanosensitive responses in human mesenchymal stem cells. Small (Weinheim an der Bergstrasse, Germany), 2020. 16(10): p. e1905422.
Nakamoto, T., et al. (2014). Influence of micropattern width on differentiation of human mesenchymal stem cells to vascular smooth muscle cells. Colloids and Surfaces. B, Biointerfaces, 122, 316–323.
Zhou, W., et al. (2020). Effect of micropore/microsphere topography and a silicon-incorporating modified titanium plate surface on the adhesion and osteogenic differentiation of BMSCs. Artif Cells Nanomed Biotechnol, 48(1), 230–241.
Wang, M., et al., Microarray analysis reveals that lncRNA PWRN1–209 promotes human bone marrow mesenchymal stem cell osteogenic differentiation on microtopography titanium surface in vitro. J Biomed Mater Res B Appl Biomater, 2020.
Ruminski, S., et al. (2019). Osteogenic differentiation of human adipose-derived stem cells in 3D conditions - comparison of spheroids and polystyrene scaffolds. European Cells & Materials, 37, 382–401.
Zhou, W., et al. (2019). Effect of titanium implants with coatings of different pore sizes on adhesion and osteogenic differentiation of BMSCs. Artif Cells Nanomed Biotechnol, 47(1), 290–299.
Docheva, D., et al. (2007). Human mesenchymal stem cells in contact with their environment: Surface characteristics and the integrin system. Journal of Cellular and Molecular Medicine, 11(1), 21–38.
Lopes, H. B., et al. (2019). Participation of integrin beta3 in osteoblast differentiation induced by titanium with nano or microtopography. Journal of Biomedical Materials Research. Part A, 107(6), 1303–1313.
Lopes, H. B., et al. (2019). Titanium with nanotopography induces osteoblast differentiation through regulation of integrin αV. Journal of Cellular Biochemistry, 120(10), 16723–16732.
Olivares-Navarrete, R., et al. (2008). Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc Natl Acad Sci U S A, 105(41), 15767–15772.
Wang, W., et al. (2012). The role of the Wnt/β-catenin pathway in the effect of implant topography on MG63 differentiation. Biomaterials, 33(32), 7993–8002.
Vining, K. H., & Mooney, D. J. (2017). Mechanical forces direct stem cell behaviour in development and regeneration. Nature Reviews Molecular Cell Biology, 18(12), 728–742.
Sun, M., et al. (2018). Effects of matrix stiffness on the morphology, adhesion, proliferation and osteogenic differentiation of mesenchymal stem cells. International Journal of Medical Sciences, 15(3), 257–268.
Gerardo, H., et al. (2019). Soft culture substrates favor stem-like cellular phenotype and facilitate reprogramming of human mesenchymal stem/stromal cells (hMSCs) through mechanotransduction. Science and Reports, 9(1), 9086.
Wu, L., et al., Thermoresponsive Stiffness Softening of Hierarchically Porous Nanohybrid Membranes Promotes Niches for Mesenchymal Stem Cell Differentiation. Adv Healthc Mater, 2019. 8(10): p. e1801556.
Olivares-Navarrete, R., et al., Substrate stiffness controls osteoblastic and chondrocytic differentiation of mesenchymal stem cells without exogenous stimuli. PLoS One, 2017. 12(1): p. e0170312.
Gungordu, H. I., et al. (2019). Effect of mechanical loading and substrate elasticity on the osteogenic and adipogenic differentiation of mesenchymal stem cells. Journal of Tissue Engineering and Regenerative Medicine, 13(12), 2279–2290.
Wei, D., et al. (2020). Mechanics-controlled dynamic cell niches guided osteogenic differentiation of stem cells via preserved cellular mechanical memory. ACS Applied Materials & Interfaces, 12(1), 260–274.
Zhan, X. (2020). Effect of matrix stiffness and adhesion ligand density on chondrogenic differentiation of mesenchymal stem cells. Journal of Biomedical Materials Research. Part A, 108(3), 675–683.
Ledo, A. M., et al. (2020). Extracellular matrix mechanics regulate transfection and SOX9-directed differentiation of mesenchymal stem cells. Acta biomaterialia, 110, 153–163.
Sun, M., et al. (2018). Extracellular matrix stiffness controls osteogenic differentiation of mesenchymal stem cells mediated by integrin α5. Stem cell research & therapy, 9(1), 52.
Sun, Y., et al. (2020). Matrix stiffness regulates myocardial differentiation of human umbilical cord mesenchymal stem cells. Aging, 13(2), 2231–2250.
Park, J. S., et al. (2011). The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials, 32(16), 3921–3930.
Kechagia, J. Z., Ivaska, J., & Roca-Cusachs, P. (2019). Integrins as biomechanical sensors of the microenvironment. Nature Reviews Molecular Cell Biology, 20(8), 457–473.
Wan, W., et al. (2019). Synergistic effect of matrix stiffness and inflammatory factors on osteogenic differentiation of MSC. Biophysical Journal, 117(1), 129–142.
Jiang, P., Mao, Z., & Gao, C. (2015). Combinational effect of matrix elasticity and alendronate density on differentiation of rat mesenchymal stem cells. Acta Biomaterialia, 19, 76–84.
Hou, Y., et al. (2020). Surface roughness and substrate stiffness synergize to drive cellular mechanoresponse. Nano letters, 20(1), 748–757.
Ventre, M., et al. (2019). Aligned fibrous decellularized cell derived matrices for mesenchymal stem cell amplification. Journal of Biomedical Materials Research. Part A, 107(11), 2536–2546.
Pattappa, G., et al. (2019). Cells under pressure - the relationship between hydrostatic pressure and mesenchymal stem cell chondrogenesis. European Cells & Materials, 37, 360–381.
Argentati, C., et al., Insight into Mechanobiology: How Stem Cells Feel Mechanical Forces and Orchestrate Biological Functions. Int J Mol Sci, 2019. 20(21).
Liu, C., Kaneko, S., & Soma, K. (2008). Expression of integrinalpha5beta1, focal adhesion kinase and integrin-linked kinase in rat condylar cartilage during mandibular lateral displacement. Archives of Oral Biology, 53(8), 701–708.
Steward, A. J., et al. (2012). Cell-matrix interactions regulate mesenchymal stem cell response to hydrostatic pressure. Acta Biomaterialia, 8(6), 2153–2159.
Tang, X., et al. (2017). Influence of hydrodynamic pressure on the proliferation and osteogenic differentiation of bone mesenchymal stromal cells seeded on polyurethane scaffolds. Journal of Biomedical Materials Research. Part A, 105(12), 3445–3455.
Angele, P., et al. (2003). Cyclic hydrostatic pressure enhances the chondrogenic phenotype of human mesenchymal progenitor cells differentiated in vitro. Journal of Orthopaedic Research, 21(3), 451–457.
Stavenschi, E., et al. (2018). Physiological cyclic hydrostatic pressure induces osteogenic lineage commitment of human bone marrow stem cells: A systematic study. Stem Cell Research & Therapy, 9(1), 276.
Huang, C., & Ogawa, R. (2012). Effect of hydrostatic pressure on bone regeneration using human mesenchymal stem cells. Tissue Engineering Part A, 18(19–20), 2106–2113.
Stavenschi, E., & Hoey, D. A. (2019). Pressure-induced mesenchymal stem cell osteogenesis is dependent on intermediate filament remodeling. Faseb Journal, 33(3), 4178–4187.
Cheng, B., et al. (2019). The role of anthrax toxin protein receptor 1 as a new mechanosensor molecule and its mechanotransduction in BMSCs under hydrostatic pressure. Science and Reports, 9(1), 12642.
Baker, B. M., et al. (2011). Dynamic tensile loading improves the functional properties of mesenchymal stem cell-laden nanofiber-based fibrocartilage. Tissue Engineering Part A, 17(9–10), 1445–1455.
Rui, Y. F., et al. (2011). Mechanical loading increased BMP-2 expression which promoted osteogenic differentiation of tendon-derived stem cells. Journal of Orthopaedic Research, 29(3), 390–396.
McMahon, L. A., Campbell, V. A., & Prendergast, P. J. (2008). Involvement of stretch-activated ion channels in strain-regulated glycosaminoglycan synthesis in mesenchymal stem cell-seeded 3D scaffolds. Journal of Biomechanics, 41(9), 2055–2059.
Zeng, Q., et al. (2015). Integrin-β1, not integrin-β5, mediates osteoblastic differentiation and ECM formation promoted by mechanical tensile strain. Biological Research, 48(1), 25.
Grier, W. G., Moy, A. S., & Harley, B. A. (2017). Cyclic tensile strain enhances human mesenchymal stem cell Smad 2/3 activation and tenogenic differentiation in anisotropic collagen-glycosaminoglycan scaffolds. European Cells & Materials, 33, 227–239.
Su, X., et al. (2020). Effects of dynamic radial tensile stress on fibrocartilage differentiation of bone marrow mesenchymal stem cells. Biomedical Engineering Online, 19(1), 8.
Chen, J. and X. Wu, Cyclic tensile strain promotes chondrogenesis of bone marrow-derived mesenchymal stem cells by increasing miR-365 expression. Life Sci, 2019. 232: p. 116625.
Yu, H., et al. (2019). Expression of HIF-1 alpha in cycling stretch-induced osteogenic differentiation of bone mesenchymal stem cells. Molecular Medicine Reports, 20(5), 4489–4498.
Xie, Y., et al., Proper mechanical stimulation improve the chondrogenic differentiation of mesenchymal stem cells: Improve the viscoelasticity and chondrogenic phenotype. Biomed Pharmacother, 2019. 115: p. 108935.
Ouyang, X., Y. Xie, and G. Wang, Mechanical stimulation promotes the proliferation and the cartilage phenotype of mesenchymal stem cells and chondrocytes co-cultured in vitro. Biomed Pharmacother, 2019. 117: p. 109146.
Paddillaya, N., et al. (2019). Biophysics of cell-substrate interactions under shear. Front Cell Dev Biol, 7, 251.
Han, P., et al. (2019). Five piconewtons: the difference between osteogenic and adipogenic fate choice in human mesenchymal stem cells. ACS Nano, 13(10), 11129–11143.
Xanthis, I., et al., β1 integrin is a sensor of blood flow direction. J Cell Sci, 2019. 132(11).
Arnsdorf, E. J., et al. (2009). Mechanically induced osteogenic differentiation–the role of RhoA, ROCKII and cytoskeletal dynamics. Journal of Cell Science, 122(Pt 4), 546–553.
Liu, X., Zhang, X., & Lee, I. (2010). A quantitative study on morphological responses of osteoblastic cells to fluid shear stress. Acta Biochimica et Biophysica Sinica (Shanghai), 42(3), 195–201.
Sonam, S., et al. (2016). Cell contractility arising from topography and shear flow determines human mesenchymal stem cell fate. Science and Reports, 6, 20415.
Kupcsik, L., et al. (2010). Improving chondrogenesis: Potential and limitations of SOX9 gene transfer and mechanical stimulation for cartilage tissue engineering. Tissue Engineering Part A, 16(6), 1845–1855.
Zhang, C., et al. (2018). Space microgravity drives transdifferentiation of human bone marrow-derived mesenchymal stem cells from osteogenesis to adipogenesis. The FASEB Journal, 32(8), 4444–4458.
Huang, C. Y., et al. (2004). Effects of cyclic compressive loading on chondrogenesis of rabbit bone-marrow derived mesenchymal stem cells. Stem Cells, 22(3), 313–323.
Haugh, M. G., et al. (2011). Temporal and spatial changes in cartilage-matrix-specific gene expression in mesenchymal stem cells in response to dynamic compression. Tissue Engineering Part A, 17(23–24), 3085–3093.
Ge, Y., et al., Effects of Mechanical Compression on Chondrogenesis of Human Synovium-Derived Mesenchymal Stem Cells in Agarose Hydrogel. Frontiers in bioengineering and biotechnology, 2021. 9: p. 697281.
Zhang, T., et al. (2015). Cross-talk between TGF-beta/SMAD and integrin signaling pathways in regulating hypertrophy of mesenchymal stem cell chondrogenesis under deferral dynamic compression. Biomaterials, 38, 72–85.
Li, C.W., et al., Mechanically induced formation and maturation of 3D-matrix adhesions (3DMAs) in human mesenchymal stem cells. Biomaterials, 2020. 258: p. 120292.
Lin, X., et al., The Impact of Spaceflight and Simulated Microgravity on Cell Adhesion. Int J Mol Sci, 2020. 21(9).
Lü, D., et al. (2019). Microgravity-induced hepatogenic differentiation of rBMSCs on board the SJ-10 satellite. The FASEB Journal, 33(3), 4273–4286.
Luo, B. H., Carman, C. V., & Springer, T. A. (2007). Structural basis of integrin regulation and signaling. Annual Review of Immunology, 25, 619–647.
Calderwood, D. A., Campbell, I. D., & Critchley, D. R. (2013). Talins and kindlins: Partners in integrin-mediated adhesion. Nature Reviews Molecular Cell Biology, 14(8), 503–517.
Steward, A. J., Wagner, D. R., & Kelly, D. J. (2014). Exploring the roles of integrin binding and cytoskeletal reorganization during mesenchymal stem cell mechanotransduction in soft and stiff hydrogels subjected to dynamic compression. Journal of the mechanical behavior of biomedical materials, 38, 174–182.
Shahmoradi, S. R., et al. (2019). Induction of Chondrogenic Differentiation in Human Mesenchymal Stem Cells Cultured on Human Demineralized Bone Matrix Scaffold under Hydrostatic Pressure. Tissue Eng Regen Med, 16(1), 69–80.
Kang, J. M., et al. (2020). Characterization of cell signaling, morphology, and differentiation potential of human mesenchymal stem cells based on cell adhesion mechanism. Journal of Cellular Physiology, 235(10), 6915–6928.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant No. 31201052). The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
Funding
This study was funded by the National Natural Science Foundation of China (Grant No. 31201052). The authors confirm independence from the sponsors; the content of the article has not been influenced by the sponsors.
Author information
Authors and Affiliations
Contributions
Lei Wang, Fuwen Zheng, Ruixue Song, and Lequan Zhuang participated in the writing. Fuwen Zheng and Lequan Zhuang designed and prepared the figures. Fuwen Zheng and Ruixue Song designed and prepared the tables. Fuwen Zheng and Jian Suo was in charge of proofreading manuscripts. Lisha Li, Jian Suo and Ming Yang designed and polished the paper.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Open Access
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third-party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The fate of cell is regulated.
Rights and permissions
About this article
Cite this article
Wang, L., Zheng, F., Song, R. et al. Integrins in the Regulation of Mesenchymal Stem Cell Differentiation by Mechanical Signals. Stem Cell Rev and Rep 18, 126–141 (2022). https://doi.org/10.1007/s12015-021-10260-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10260-5