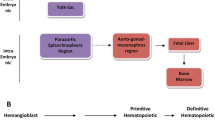
Abstract
As with most organ systems that undergo continuous generation and maturation during the transition from fetal to adult life, the hematopoietic and immune systems also experience dynamic changes. Such changes lead to many unique features in blood cell function and immune responses in early childhood. The blood cells and immune cells in neonates are a mixture of fetal and adult origin due to the co-existence of both fetal and adult types of hematopoietic stem cells (HSCs) and progenitor cells (HPCs). Fetal blood and immune cells gradually diminish during maturation of the infant and are almost completely replaced by adult types of cells by 3 to 4 weeks after birth in mice. Such features in early childhood are associated with unique features of hematopoietic and immune diseases, such as leukemia, at these developmental stages. Therefore, understanding the cellular and molecular mechanisms by which hematopoietic and immune changes occur throughout ontogeny will provide useful information for the study and treatment of pediatric blood and immune diseases. In this review, we summarize the most recent studies on hematopoietic initiation during early embryonic development, the expansion of both fetal and adult types of HSCs and HPCs in the fetal liver and fetal bone marrow stages, and the shift from fetal to adult hematopoiesis/immunity during neonatal/infant development. We also discuss the contributions of fetal types of HSCs/HPCs to childhood leukemias.
Graphical Abstract





Similar content being viewed by others
Abbreviations
- AGM:
-
Aorta-gonad-mesonephros
- Angptl2:
-
Angiopoietin-like 2
- AMkL:
-
Acute megakaryocytic type
- AML:
-
Acute myeloid leukemia
- ALL:
-
Acute lymphoblastic type
- BM:
-
Bone marrow
- CSF1:
-
Colony-stimulating factor 1
- CXCL12:
-
C-X-C Motif Chemokine Ligand 12
- drHSCs:
-
Developmentally-restricted HSCs
- DS:
-
Down Syndrome
- E:
-
Embryonic day
- ECs:
-
Endothelial cells
- EPO:
-
Erythropoietin
- EMPs:
-
Erythro-myeloid progenitors
- ECM:
-
Extracellular matrix
- FL:
-
Fetal liver
- HSCs:
-
Hematopoietic stem cells
- HPCs:
-
Hematopoietic progenitor cells
- Hb:
-
Hemoglobin
- imHSCs:
-
Immature HSC
- IGF2:
-
Insulin-like growth factor 2
- IL:
-
Interleukin
- IFN:
-
Interferon
- KITL:
-
KIT ligand
- LMPs:
-
Lympho-myeloid progenitors
- Mφs:
-
Macrophages
- Mks:
-
Megakaryocytes
- MLL:
-
Mixed lineage leukemia
- ELPs:
-
Early lymphoid progenitors
- PB:
-
Peripheral blood
- PCW:
-
Post-conception weeks
- pri-EPs:
-
Primitive erythroid progenitors
- RBCs:
-
Red blood cells
- THPO:
-
Thrombopoietin
- TSLP:
-
Thymic stromal-derived lymphopoietin
- TMD:
-
Transient myeloproliferative disorder
- vWF:
-
von Willebrand factor
- YS:
-
Yolk Sac
References
Kikuchi, K., & Kondo, M. (2006). Developmental switch of mouse hematopoietic stem cells from fetal to adult type occurs in bone marrow after birth. Proceedings of the National Academy of Sciences, 103(47), 17852–17857.
Bowie, M. B., Kent, D. G., Dykstra, B., McKnight, K. D., McCaffrey, L., Hoodless, P. A., & Eaves, C. J. (2007). Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proceedings of the National Academy of Sciences, 104(14), 5878–5882.
Bowie, M. B., McKnight, K. D., Kent, D. G., McCaffrey, L., Hoodless, P. A., & Eaves, C. J. (2006). Hematopoietic stem cells proliferate until after birth and show a reversible phase-specific engraftment defect. The Journal of Clinical Investigation, 116(10), 2808–2816.
Gothert, J. R., Gustin, S. E., Hall, M. A., Green, A. R., Gottgens, B., Izon, D. J., & Begley, C. G. (2005). In vivo fate-tracing studies using the Scl stem cell enhancer: Embryonic hematopoietic stem cells significantly contribute to adult hematopoiesis. Blood, 105(7), 2724–2732.
Morrison, S. J., Hemmati, H. D., Wandycz, A. M., & Weissman, I. L. (1995). The purification and characterization of fetal liver hematopoietic stem cells. Proceedings of the National Academy of Sciences, 92(22), 10302–10306.
Yuan, J., Nguyen, C. K., Liu, X., Kanellopoulou, C., & Muljo, S. A. (2012). Lin28b reprograms adult bone marrow hematopoietic progenitors to mediate fetal-like lymphopoiesis. Science, 335(6073), 1195–1200.
Copley, M. R., Babovic, S., Benz, C., Knapp, D. J., Beer, P. A., Kent, D. G., Wohrer, S., Treloar, D. Q., Day, C., Rowe, K., et al. (2013). The Lin28b-let-7-Hmga2 axis determines the higher self-renewal potential of fetal haematopoietic stem cells. Nature Cell Biology, 15(8), 916–925.
Benz, C., Copley, M. R., Kent, D. G., Wohrer, S., Cortes, A., Aghaeepour, N., Ma, E., Mader, H., Rowe, K., Day, C., et al. (2012). Hematopoietic stem cell subtypes expand differentially during development and display distinct lymphopoietic programs. Cell Stem Cell, 10(3), 273–283.
Ghosn, E. E., Yamamoto, R., Hamanaka, S., Yang, Y., Herzenberg, L. A., Nakauchi, H., & Herzenberg, L. A. (2012). Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proceedings of the National Academy of Sciences, 109(14), 5394–5398.
Barber, C. L., Montecino-Rodriguez, E., & Dorshkind, K. (2011). Reduced production of B-1-specified common lymphoid progenitors results in diminished potential of adult marrow to generate B-1 cells. Proceedings of the National Academy of Science, 108(33), 13700–13704.
Yan, H., Hale, J., Jaffray, J., Li, J., Wang, Y., Huang, Y., An, X., Hillyer, C., Wang, N., Kinet, S., et al. (2018). Developmental differences between neonatal and adult human erythropoiesis. American Journal of Hematology, 93(4), 494–503.
Birger, Y., Goldberg, L., Chlon, T. M., Goldenson, B., Muler, I., Schiby, G., Jacob-Hirsch, J., Rechavi, G., Crispino, J. D., & Izraeli, S. (2013). Perturbation of fetal hematopoiesis in a mouse model of Down syndrome’s transient myeloproliferative disorder. Blood, 122(6), 988–998.
Okeyo-Owuor, T., Li, Y., Patel, R. M., Yang, W., Casey, E. B., Cluster, A. S., Porter, S. N., Bryder, D., & Magee, J. A. (2019). The efficiency of murine MLL-ENL-driven leukemia initiation changes with age and peaks during neonatal development. Blood advances, 3(15), 2388–2399.
Porter, S. N., Cluster, A. S., Yang, W., Busken, K. A., Patel, R. M., Ryoo, J., & Magee, J. A. (2016). Fetal and neonatal hematopoietic progenitors are functionally and transcriptionally resistant to Flt3-ITD mutations. eLife, 5, e18882.
Li, Y., Kong, W., Yang, W., Patel, R. M., Casey, E. B., Okeyo-Owuor, T., White, J. M., Porter, S. N., Morris, S. A., & Magee, J. A. (2020). Single-cell analysis of neonatal HSC ontogeny reveals gradual and uncoordinated transcriptional reprogramming that begins before birth. Cell Stem Cell, 27(5), 732-747 e737.
Parekh, C., & Crooks, G. M. (2013). Critical differences in hematopoiesis and lymphoid development between humans and mice. Journal of Clinical Immunology, 33(4), 711–715.
Ivanovs, A., Rybtsov, S., Ng, E. S., Stanley, E. G., Elefanty, A. G., & Medvinsky, A. (2017). Human haematopoietic stem cell development: From the embryo to the dish. Development, 144(13), 2323–2337.
Bertrand, J. Y., Jalil, A., Klaine, M., Jung, S., Cumano, A., & Godin, I. (2005). Three pathways to mature macrophages in the early mouse yolk sac. Blood, 106(9), 3004–3011.
McGrath, K. E., Frame, J. M., Fegan, K. H., Bowen, J. R., Conway, S. J., Catherman, S. C., Kingsley, P. D., Koniski, A. D., & Palis, J. (2015). Distinct sources of hematopoietic progenitors emerge before HSCs and provide functional blood cells in the mammalian embryo. Cell reports, 11(12), 1892–1904.
Palis, J., Robertson, S., Kennedy, M., Wall, C., & Keller, G. (1999). Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development, 126(22), 5073–5084.
Kingsley, P. D., Malik, J., Emerson, R. L., Bushnell, T. P., McGrath, K. E., Bloedorn, L. A., Bulger, M., & Palis, J. (2006). “Maturational” globin switching in primary primitive erythroid cells. Blood, 107(4), 1665–1672.
Tober, J., Koniski, A., McGrath, K. E., Vemishetti, R., Emerson, R., de Mesy-Bentley, K. K., Waugh, R., & Palis, J. (2007). The megakaryocyte lineage originates from hemangioblast precursors and is an integral component both of primitive and of definitive hematopoiesis. Blood, 109(4), 1433–1441.
Frame, J. M., Fegan, K. H., Conway, S. J., McGrath, K. E., & Palis, J. (2016). Definitive hematopoiesis in the yolk sac emerges from wnt-responsive hemogenic endothelium independently of circulation and arterial identity. Stem Cells, 34(2), 431–444.
Boiers, C., Carrelha, J., Lutteropp, M., Luc, S., Green, J. C., Azzoni, E., Woll, P. S., Mead, A. J., Hultquist, A., Swiers, G., et al. (2013). Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell, 13(5), 535–548.
Inlay, M. A., Serwold, T., Mosley, A., Fathman, J. W., Dimov, I. K., Seita, J., & Weissman, I. L. (2014). Identification of multipotent progenitors that emerge prior to hematopoietic stem cells in embryonic development. Stem cell reports, 2(4), 457–472.
Kasaai, B., Caolo, V., Peacock, H. M., Lehoux, S., Gomez-Perdiguero, E., Luttun, A., & Jones, E. A. (2017). Erythro-myeloid progenitors can differentiate from endothelial cells and modulate embryonic vascular remodeling. Scientific Reports, 7, 43817.
McGrath, K. E., Frame, J. M., & Palis, J. (2015). Early hematopoiesis and macrophage development. Seminars in Immunology, 27(6), 379–387.
Gentek, R., Ghigo, C., Hoeffel, G., Bulle, M. J., Msallam, R., Gautier, G., Launay, P., Chen, J., Ginhoux, F., & Bajenoff, M. (2018). Hemogenic endothelial fate mapping reveals dual developmental origin of mast cells. Immunity, 48(6), 1160-1171 e1165.
Dege, C., Fegan, K. H., Creamer, J. P., Berrien-Elliott, M. M., Luff, S. A., Kim, D., Wagner, J. A., Kingsley, P. D., McGrath, K. E., Fehniger, T. A., et al. (2020). Potently cytotoxic natural killer cells initially emerge from Erythro-Myeloid progenitors during mammalian development. Developmental Cell, 53(2), 229-239 e227.
Li, Z., Liu, S., Xu, J., Zhang, X., Han, D., Liu, J., Xia, M., Yi, L., Shen, Q., Xu, S., et al. (2018). Adult connective tissue-resident mast cells originate from late erythro-myeloid progenitors. Immunity, 49(4), 640-653 e645.
Schneider, C., Lee, J., Koga, S., Ricardo-Gonzalez, R. R., Nussbaum, J. C., Smith, L. K., Villeda, S. A., Liang, H. E., & Locksley, R. M. (2019). Tissue-Resident Group 2 innate lymphoid cells differentiate by layered ontogeny and in situ perinatal priming. Immunity, 50(6), 1425-1438 e1425.
Gentek, R., Ghigo, C., Hoeffel, G., Jorquera, A., Msallam, R., Wienert, S., Klauschen, F., Ginhoux, F., & Bajenoff, M. (2018). Epidermal gammadelta T cells originate from yolk sac hematopoiesis and clonally self-renew in the adult. Journal of Experimental Medicine, 215(12), 2994–3005.
McGrath, K. E., Frame, J. M., Fromm, G. J., Koniski, A. D., Kingsley, P. D., Little, J., Bulger, M., & Palis, J. (2011). A transient definitive erythroid lineage with unique regulation of the beta-globin locus in the mammalian embryo. Blood, 117(17), 4600–4608.
Bian, Z., Gong, Y., Huang, T., Lee, C. Z. W., Bian, L., Bai, Z., Shi, H., Zeng, Y., Liu, C., He, J., et al. (2020). Deciphering human macrophage development at single-cell resolution. Nature, 582(7813), 571–576.
Popescu, D. M., Botting, R. A., Stephenson, E., Green, K., Webb, S., Jardine, L., Calderbank, E. F., Polanski, K., Goh, I., Efremova, M., et al. (2019). Decoding human fetal liver haematopoiesis. Nature, 574(7778), 365–371.
Werner, Y., Mass, E., Ashok Kumar, P., Ulas, T., Handler, K., Horne, A., Klee, K., Lupp, A., Schutz, D., Saaber, F., et al. (2020). Cxcr4 distinguishes HSC-derived monocytes from microglia and reveals monocyte immune responses to experimental stroke. Nature Neuroscience, 23(3), 351–362.
Yoshimoto, M., Porayette, P., Glosson, N. L., Conway, S. J., Carlesso, N., Cardoso, A. A., Kaplan, M. H., & Yoder, M. C. (2012). Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood, 119(24), 5706–5714.
Lin, Y., Kobayashi, M., Azevedo Portilho, N., Mishra, A., Gao, H., Liu, Y., Wenzel, P., Davis, B., Yoder, M. C., & Yoshimoto, M. (2019). Long-term engraftment of ESC-Derived B-1 progenitor cells supports HSC-independent lymphopoiesis. Stem cell reports, 12(3), 572–583.
Luis, T. C., Luc, S., Mizukami, T., Boukarabila, H., Thongjuea, S., Woll, P. S., Azzoni, E., Giustacchini, A., Lutteropp, M., Bouriez-Jones, T., et al. (2016). Initial seeding of the embryonic thymus by immune-restricted lympho-myeloid progenitors. Nature Immunology, 17(12), 1424–1435.
Beaudin, A. E., Boyer, S. W., Perez-Cunningham, J., Hernandez, G. E., Derderian, S. C., Jujjavarapu, C., Aaserude, E., MacKenzie, T., & Forsberg, E. C. (2016). A transient developmental hematopoietic stem cell gives rise to innate-like B and T Cells. Cell Stem Cell, 19(6), 768–783.
Adolfsson, J., Mansson, R., Buza-Vidas, N., Hultquist, A., Liuba, K., Jensen, C. T., Bryder, D., Yang, L., Borge, O. J., Thoren, L. A., et al. (2005). Identification of Flt3+ lympho-myeloid stem cells lacking erythro-megakaryocytic potential a revised road map for adult blood lineage commitment. Cell, 121(2), 295–306.
Yamane, T., Hosen, N., Yamazaki, H., & Weissman, I. L. (2009). Expression of AA4.1 marks lymphohematopoietic progenitors in early mouse development. Proceedings of the National Academy of Sciences U S A, 106(22), 8953–8958.
Ito, C., Yamazaki, H., & Yamane, T. (2013). Earliest hematopoietic progenitors at embryonic day 9 preferentially generate B-1 B cells rather than follicular B or marginal zone B cells. Biochemical and Biophysical Research Communications, 437(2), 307–313.
Yamane, T., Ito, C., Washino, A., Isono, K., & Yamazaki, H. (2017). Repression of primitive erythroid program is critical for the initiation of multi-lineage hematopoiesis in mouse development. Journal of Cellular Physiology, 232(2), 323–330.
Medvinsky, A., & Dzierzak, E. (1996). Definitive hematopoiesis is autonomously initiated by the AGM region. Cell, 86(6), 897–906.
North, T. E., de Bruijn, M. F., Stacy, T., Talebian, L., Lind, E., Robin, C., Binder, M., Dzierzak, E., & Speck, N. A. (2002). Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity, 16(5), 661–672.
Bertrand, J. Y., Chi, N. C., Santoso, B., Teng, S., Stainier, D. Y., & Traver, D. (2010). Haematopoietic stem cells derive directly from aortic endothelium during development. Nature, 464(7285), 108–111.
Boisset, J. C., van Cappellen, W., Andrieu-Soler, C., Galjart, N., Dzierzak, E., & Robin, C. (2010). In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature, 464(7285), 116–120.
Chen, M. J., Yokomizo, T., Zeigler, B. M., Dzierzak, E., & Speck, N. A. (2009). Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature, 457(7231), 887–891.
Eilken, H. M., Nishikawa, S., & Schroeder, T. (2009). Continuous single-cell imaging of blood generation from haemogenic endothelium. Nature, 457(7231), 896–900.
Lancrin, C., Sroczynska, P., Stephenson, C., Allen, T., Kouskoff, V., & Lacaud, G. (2009). The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature, 457(7231), 892–895.
Zovein, A. C., Hofmann, J. J., Lynch, M., French, W. J., Turlo, K. A., Yang, Y., Becker, M. S., Zanetta, L., Dejana, E., Gasson, J. C., et al. (2008). Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell, 3(6), 625–636.
Schulz, C., Gomez Perdiguero, E., Chorro, L., Szabo-Rogers, H., Cagnard, N., Kierdorf, K., Prinz, M., Wu, B., Jacobsen, S. E., Pollard, J. W., et al. (2012). A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science, 336(6077), 86–90.
Tober, J., McGrath, K. E., & Palis, J. (2008). Primitive erythropoiesis and megakaryopoiesis in the yolk sac are independent of c-myb. Blood, 111(5), 2636–2639.
Rybtsov, S., Sobiesiak, M., Taoudi, S., Souilhol, C., Senserrich, J., Liakhovitskaia, A., Ivanovs, A., Frampton, J., Zhao, S., & Medvinsky, A. (2011). Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. Journal of Experimental Medicine, 208(6), 1305–1315.
Rybtsov, S., Batsivari, A., Bilotkach, K., Paruzina, D., Senserrich, J., Nerushev, O., & Medvinsky, A. (2014). Tracing the origin of the HSC hierarchy reveals an SCF-dependent, IL-3-independent CD43(-) embryonic precursor. Stem Cell Reports, 3(3), 489–501.
Rybtsov, S., Ivanovs, A., Zhao, S., & Medvinsky, A. (2016). Concealed expansion of immature precursors underpins acute burst of adult HSC activity in foetal liver. Development, 143(8), 1284–1289.
Ye, H., Wang, X., Li, Z., Zhou, F., Li, X., Ni, Y., Zhang, W., Tang, F., Liu, B., & Lan, Y. (2017). Clonal analysis reveals remarkable functional heterogeneity during hematopoietic stem cell emergence. Cell Research, 27(8), 1065–1068.
Ganuza, M., Hall, T., Finkelstein, D., Chabot, A., Kang, G., & McKinney-Freeman, S. (2017). Lifelong haematopoiesis is established by hundreds of precursors throughout mammalian ontogeny. Nature Cell Biology, 19(10), 1153–1163.
Henninger, J., Santoso, B., Hans, S., Durand, E., Moore, J., Mosimann, C., Brand, M., Traver, D., & Zon, L. (2017). Clonal fate mapping quantifies the number of haematopoietic stem cells that arise during development. Nature Cell Biology, 19(1), 17–27.
Pronk, C. J., Rossi, D. J., Mansson, R., Attema, J. L., Norddahl, G. L., Chan, C. K., Sigvardsson, M., Weissman, I. L., & Bryder, D. (2007). Elucidation of the phenotypic, functional, and molecular topography of a myeloerythroid progenitor cell hierarchy. Cell Stem Cell, 1(4), 428–442.
Gekas, C., Dieterlen-Lievre, F., Orkin, S. H., & Mikkola, H. K. (2005). The placenta is a niche for hematopoietic stem cells. Developmental Cell, 8, 365–375.
Robin, C., Bollerot, K., Mendes, S., Haak, E., Crisan, M., Cerisoli, F., Lauw, I., Kaimakis, P., Jorna, R., Vermeulen, M., et al. (2009). Human placenta is a potent hematopoietic niche containing hematopoietic stem and progenitor cells throughout development. Cell Stem Cell, 5(4), 385–395.
Ottersbach, K., & Dzierzak, E. (2005). The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Developmental Cell, 8(3), 377–387.
Barcena, A., Muench, M. O., Kapidzic, M., & Fisher, S. J. (2009). A new role for the human placenta as a hematopoietic site throughout gestation. Reproductive Sciences, 16(2), 178–187.
Barcena, A., Kapidzic, M., Muench, M. O., Gormley, M., Scott, M. A., Weier, J. F., Ferlatte, C., & Fisher, S. J. (2009). The human placenta is a hematopoietic organ during the embryonic and fetal periods of development. Developmental Biology, 327(1), 24–33.
Rhodes, K. E., Gekas, C., Wang, Y., Lux, C. T., Francis, C. S., Chan, D. N., Conway, S., Orkin, S. H., Yoder, M. C., & Mikkola, H. K. (2008). The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell, 2(3), 252–263.
Mucenski, M. L., McLain, K., Kier, A. B., Swerdlow, S. H., Schreiner, C. M., Miller, T. A., Pietryga, D. W., Scott, W. J., Jr., & Potter, S. S. (1991). A functional c-myb gene is required for normal murine fetal hepatic hematopoiesis. Cell, 65(4), 677–689.
Ginhoux, F., Greter, M., Leboeuf, M., Nandi, S., See, P., Gokhan, S., Mehler, M. F., Conway, S. J., Ng, L. G., Stanley, E. R., et al. (2010). Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science, 330(6005), 841–845.
Sheng, J., Ruedl, C., & Karjalainen, K. (2015). Most tissue-resident macrophages except microglia are derived from fetal hematopoietic stem cells. Immunity, 43(2), 382–393.
Samokhvalov, I. M., Samokhvalova, N. I., & Nishikawa, S. (2007). Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature, 446(7139), 1056–1061.
Lux, C. T., Yoshimoto, M., McGrath, K., Conway, S. J., Palis, J., & Yoder, M. C. (2008). All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood, 111(7), 3435–3438.
Gomez Perdiguero, E., Klapproth, K., Schulz, C., Busch, K., Azzoni, E., Crozet, L., Garner, H., Trouillet, C., de Bruijn, M. F., Geissmann, F., et al. (2015). Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature, 518(7540), 547–551.
Kashem, S. W., Haniffa, M., & Kaplan, D. H. (2017). Antigen-Presenting Cells in the Skin. Annual Review of Immunology, 35, 469–499.
Ayres-Silva Jde, P., Manso, P. P., Madeira, M. R., Pelajo-Machado, M., & Lenzi, H. L. (2011). Sequential morphological characteristics of murine fetal liver hematopoietic microenvironment in Swiss Webster mice. Cell and Tissue Research, 344(3), 455–469.
Miles, C., Sanchez, M. J., Sinclair, A., & Dzierzak, E. (1997). Expression of the Ly-6.E1 (Sca-1) transgene in adult hematopoietic stem cells and the developing mouse embryo. Development, 124(2), 537–547.
Brouard, N., Jost, C., Matthias, N., Albrecht, C., Egard, S., Gandhi, P., Strassel, C., Inoue, T., Sugiyama, D., Simmons, P. J., et al. (2017). A unique microenvironment in the developing liver supports the expansion of megakaryocyte progenitors. Blood Advances, 1(21), 1854–1866.
Soares-da-Silva F., Freyer L., Elsaid R., Burlen-Defranoux O., Iturri L., Sismeiro O., Pinto-do O. P., Gomez-Perdiguero E., Cumano A. (2021) Yolk sac, but not hematopoietic stem cell-derived progenitors, sustain erythropoiesis throughout murine embryonic life. Journal of Experimental Medicine, 218(4).
Chen, M. J., Li, Y., De Obaldia, M. E., Yang, Q., Yzaguirre, A. D., Yamada-Inagawa, T., Vink, C. S., Bhandoola, A., Dzierzak, E., & Speck, N. A. (2011). Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell, 9(6), 541–552.
Travnickova, J., Tran Chau, V., Julien, E., Mateos-Langerak, J., Gonzalez, C., Lelievre, E., Lutfalla, G., Tavian, M., & Kissa, K. (2015). Primitive macrophages control HSPC mobilization and definitive haematopoiesis. Nature Communications, 6, 6227.
Stremmel, C., Schuchert, R., Wagner, F., Thaler, R., Weinberger, T., Pick, R., Mass, E., Ishikawa-Ankerhold, H. C., Margraf, A., Hutter, S., et al. (2018). Yolk sac macrophage progenitors traffic to the embryo during defined stages of development. Nature Communications, 9(1), 75.
Espin-Palazon, R., Stachura, D. L., Campbell, C. A., Garcia-Moreno, D., Del Cid, N., Kim, A. D., Candel, S., Meseguer, J., Mulero, V., & Traver, D. (2014). Proinflammatory signaling regulates hematopoietic stem cell emergence. Cell, 159(5), 1070–1085.
Lee, L. K., Ghorbanian, Y., Wang, W., Wang, Y., Kim, Y. J., Weissman, I. L., Inlay, M. A., & Mikkola, H. K. A. (2016). LYVE1 Marks the divergence of yolk sac definitive hemogenic endothelium from the primitive erythroid lineage. Cell Reports, 17(9), 2286–2298.
Kim, I., He, S., Yilmaz, O. H., Kiel, M. J., & Morrison, S. J. (2006). Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood, 108(2), 737–744.
Kent, D. G., Copley, M. R., Benz, C., Wohrer, S., Dykstra, B. J., Ma, E., Cheyne, J., Zhao, Y., Bowie, M. B., Zhao, Y., et al. (2009). Prospective isolation and molecular characterization of hematopoietic stem cells with durable self-renewal potential. Blood, 113(25), 6342–6350.
Ye, M., Zhang, H., Amabile, G., Yang, H., Staber, P. B., Zhang, P., Levantini, E., Alberich-Jorda, M., Zhang, J., Kawasaki, A., et al. (2013). C/EBPa controls acquisition and maintenance of adult haematopoietic stem cell quiescence. Nature Cell Biology, 15(4), 385–394.
Li, Z., Zhou, F., Chen, D., He, W., Ni, Y., Luo, L., & Liu, B. (2013). Generation of hematopoietic stem cells from purified embryonic endothelial cells by a simple and efficient strategy. Journal of Genetics and Genomics = Yi chuan xue bao, 40(11), 557–563.
Jones, M., Chase, J., Brinkmeier, M., Xu, J., Weinberg, D. N., Schira, J., Friedman, A., Malek, S., Grembecka, J., Cierpicki, T., et al. (2015). Ash1l controls quiescence and self-renewal potential in hematopoietic stem cells. The Journal of Clinical Investigation, 125(5), 2007–2020.
Magee, J. A., Ikenoue, T., Nakada, D., Lee, J. Y., Guan, K. L., & Morrison, S. J. (2012). Temporal changes in PTEN and mTORC2 regulation of hematopoietic stem cell self-renewal and leukemia suppression. Cell Stem Cell, 11(3), 415–428.
Li, Y., Esain, V., Teng, L., Xu, J., Kwan, W., Frost, I. M., Yzaguirre, A. D., Cai, X., Cortes, M., Maijenburg, M. W., et al. (2014). Inflammatory signaling regulates embryonic hematopoietic stem and progenitor cell production. Genes and Development, 28(23), 2597–2612.
Sawamiphak, S., Kontarakis, Z., & Stainier, D. Y. (2014). Interferon gamma signaling positively regulates hematopoietic stem cell emergence. Developmental Cell, 31(5), 640–653.
Kim, P. G., Canver, M. C., Rhee, C., Ross, S. J., Harriss, J. V., Tu, H. C., Orkin, S. H., Tucker, H. O., & Daley, G. Q. (2016). Interferon-alpha signaling promotes embryonic HSC maturation. Blood, 128(2), 204–216.
Robin, C., Ottersbach, K., Durand, C., Peeters, M., Vanes, L., Tybulewicz, V., & Dzierzak, E. (2006). An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells. Developmental Cell, 11(2), 171–180.
Essers, M. A., Offner, S., Blanco-Bose, W. E., Waibler, Z., Kalinke, U., Duchosal, M. A., & Trumpp, A. (2009). IFNalpha activates dormant haematopoietic stem cells in vivo. Nature, 458(7240), 904–908.
Haas, S., Hansson, J., Klimmeck, D., Loeffler, D., Velten, L., Uckelmann, H., Wurzer, S., Prendergast, A. M., Schnell, A., Hexel, K., et al. (2015). Inflammation-induced emergency megakaryopoiesis driven by hematopoietic stem cell-like megakaryocyte progenitors. Cell Stem Cell, 17(4), 422–434.
Pietras, E. M., Lakshminarasimhan, R., Techner, J. M., Fong, S., Flach, J., Binnewies, M., & Passegue, E. (2014). Re-entry into quiescence protects hematopoietic stem cells from the killing effect of chronic exposure to type I interferons. Journal of Experimental Medicine, 211(2), 245–262.
Nomura, N., Ito, C., Ooshio, T., Tadokoro, Y., Kohno, S., Ueno, M., Kobayashi, M., Kasahara, A., Takase, Y., Kurayoshi, K., et al. (2021). Essential role of autophagy in protecting neonatal haematopoietic stem cells from oxidative stress in a p62-independent manner. Scientific Reports, 11(1), 1666.
Jung, H. E., Shim, Y. R., Oh, J. E., Oh, D. S., & Lee, H. K. (2019). The autophagy Protein Atg5 Plays a Crucial Role in the Maintenance and Reconstitution Ability of Hematopoietic Stem Cells. Immune network, 19(2), e12.
Hirsch, E., Iglesias, A., Potocnik, A. J., Hartmann, U., & Fassler, R. (1996). Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature, 380(6570), 171–175.
Potocnik, A. J., Brakebusch, C., & Fassler, R. (2000). Fetal and adult hematopoietic stem cells require beta1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity, 12(6), 653–663.
Mazo, I. B., Massberg, S., & von Andrian, U. H. (2011). Hematopoietic stem and progenitor cell trafficking. Trends in Immunology, 32(10), 493–503.
Prashad, S. L., Calvanese, V., Yao, C. Y., Kaiser, J., Wang, Y., Sasidharan, R., Crooks, G., Magnusson, M., & Mikkola, H. K. (2015). GPI-80 defines self-renewal ability in hematopoietic stem cells during human development. Cell Stem Cell, 16(1), 80–87.
Ueda, T., Yokota, T., Okuzaki, D., Uno, Y., Mashimo, T., Kubota, Y., Sudo, T., Ishibashi, T., Shingai, Y., Doi, Y., et al. (2019). Endothelial cell-selective adhesion molecule contributes to the development of definitive hematopoiesis in the fetal liver. Stem Cell Reports, 13(6), 992–1005.
Emambokus, N. R., & Frampton, J. (2003). The glycoprotein IIb molecule is expressed on early murine hematopoietic progenitors and regulates their numbers in sites of hematopoiesis. Immunity, 19(1), 33–45.
Chou, S., & Lodish, H. F. (2010). Fetal liver hepatic progenitors are supportive stromal cells for hematopoietic stem cells. Proceedings of the National Academy of Sciences U S A, 107(17), 7799–7804.
Ara, T., Tokoyoda, K., Sugiyama, T., Egawa, T., Kawabata, K., & Nagasawa, T. (2003). Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity, 19(2), 257–267.
Ciau-Uitz, A., Monteiro, R., Kirmizitas, A., & Patient, R. (2014). Developmental hematopoiesis: Ontogeny, genetic programming and conservation. Experimental Hematology, 42(8), 669–683.
Ema, H., & Nakauchi, H. (2000). Expansion of hematopoietic stem cells in the developing liver of a mouse embryo. Blood, 95(7), 2284–2288.
Chou, S., Flygare, J., & Lodish, H. F. (2013). Fetal hepatic progenitors support long-term expansion of hematopoietic stem cells. Experimental Hematology, 41(5), 479-490 e474.
Tan, K. S., Kulkeaw, K., Nakanishi, Y., & Sugiyama, D. (2017). Expression of cytokine and extracellular matrix mRNAs in fetal hepatic stellate cells. Genes to Cells, 22(9), 836–844.
Zhang, C. C., & Lodish, H. F. (2004). Insulin-like growth factor 2 expressed in a novel fetal liver cell population is a growth factor for hematopoietic stem cells. Blood, 103(7), 2513–2521.
Zheng, J., Huynh, H., Umikawa, M., Silvany, R., & Zhang, C. C. (2011). Angiopoietin-like protein 3 supports the activity of hematopoietic stem cells in the bone marrow niche. Blood, 117(2), 470–479.
Sugiyama, D., Kulkeaw, K., Mizuochi, C., Horio, Y., & Okayama, S. (2011). Hepatoblasts comprise a niche for fetal liver erythropoiesis through cytokine production. Biochemical and Biophysical Research Communications, 410(2), 301–306.
Kordes, C., Sawitza, I., Gotze, S., & Haussinger, D. (2013). Hepatic stellate cells support hematopoiesis and are liver-resident mesenchymal stem cells. Cellular Physiology and Biochemistry : International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 31(2–3), 290–304.
Liu, C., Han, T., Stachura, D. L., Wang, H., Vaisman, B. L., Kim, J., Klemke, R. L., Remaley, A. T., Rana, T. M., Traver, D., et al. (2018). Lipoprotein lipase regulates hematopoietic stem progenitor cell maintenance through DHA supply. Nature Communications, 9(1), 1310.
Iwasaki, H., Arai, F., Kubota, Y., Dahl, M., & Suda, T. (2010). Endothelial protein C receptor-expressing hematopoietic stem cells reside in the perisinusoidal niche in fetal liver. Blood, 116(4), 544–553.
Khan, J. A., Mendelson, A., Kunisaki, Y., Birbrair, A., Kou, Y., Arnal-Estape, A., Pinho, S., Ciero, P., Nakahara, F., Ma’ayan, A., et al. (2016). Fetal liver hematopoietic stem cell niches associate with portal vessels. Science, 351(6269), 176–180.
Tamplin, O. J., Durand, E. M., Carr, L. A., Childs, S. J., Hagedorn, E. J., Li, P., Yzaguirre, A. D., Speck, N. A., & Zon, L. I. (2015). Hematopoietic stem cell arrival triggers dynamic remodeling of the perivascular niche. Cell, 160(1–2), 241–252.
Gerlach, J. C., Thompson, R. L., Gridelli, B., & Schmelzer, E. (2019). Effects of delta-like noncanonical notch ligand 1 expression of human fetal liver hepatoblasts on hematopoietic progenitors. Stem Cells International, 2019, 7916275.
Sigurdsson, V., Takei, H., Soboleva, S., Radulovic, V., Galeev, R., Siva, K., Leeb-Lundberg, L. M., Iida, T., Nittono, H., & Miharada, K. (2016). Bile acids protect expanding hematopoietic stem cells from unfolded protein stress in fetal liver. Cell Stem Cell, 18(4), 522–532.
Lee Y., Leslie J., Yang Y., Ding L. (2021) Hepatic stellate and endothelial cells maintain hematopoietic stem cells in the developing liver, Journal of Experimental Medicine, 218(3).
Christensen, J. L., Wright, D. E., Wagers, A. J., & Weissman, I. L. (2004). Circulation and chemotaxis of fetal hematopoietic stem cells. PLoS Biology, 2(3), E75.
Ciriza, J., Thompson, H., Petrosian, R., Manilay, J. O., & Garcia-Ojeda, M. E. (2013). The migration of hematopoietic progenitors from the fetal liver to the fetal bone marrow: Lessons learned and possible clinical applications. Experimental Hematology, 41(5), 411–423.
Theodore, L. N., Hagedorn, E. J., Cortes, M., Natsuhara, K., Liu, S. Y., Perlin, J. R., Yang, S., Daily, M. L., Zon, L. I., & North, T. E. (2017). Distinct roles for matrix metalloproteinases 2 and 9 in embryonic hematopoietic stem cell emergence, migration, and niche colonization. Stem Cell Reports, 8(5), 1226–1241.
Papayannopoulou, T., Priestley, G. V., Nakamoto, B., Zafiropoulos, V., & Scott, L. M. (2001). Molecular pathways in bone marrow homing: Dominant role of alpha(4)beta(1) over beta(2)-integrins and selectins. Blood, 98(8), 2403–2411.
Shao, L., Chang, J., Feng, W., Wang, X., Williamson, E. A., Li, Y., Schajnovitz, A., Scadden, D., Mortensen, L. J., Lin, C. P., et al. (2018). The Wave2 scaffold Hem-1 is required for transition of fetal liver hematopoiesis to bone marrow. Nature Communications, 9(1), 2377.
Chen, Z., Borek, D., Padrick, S. B., Gomez, T. S., Metlagel, Z., Ismail, A. M., Umetani, J., Billadeau, D. D., Otwinowski, Z., & Rosen, M. K. (2010). Structure and control of the actin regulatory WAVE complex. Nature, 468(7323), 533–538.
Mizoguchi, T., Pinho, S., Ahmed, J., Kunisaki, Y., Hanoun, M., Mendelson, A., Ono, N., Kronenberg, H. M., & Frenette, P. S. (2014). Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Developmental Cell, 29(3), 340–349.
Coskun, S., Chao, H., Vasavada, H., Heydari, K., Gonzales, N., Zhou, X., de Crombrugghe, B., & Hirschi, K. K. (2014). Development of the fetal bone marrow niche and regulation of HSC quiescence and homing ability by emerging osteolineage cells. Cell Reports, 9(2), 581–590.
Cao, H., Cao, B., Heazlewood, C. K., Domingues, M., Sun, X., Debele, E., McGregor, N. E., Sims, N. A., Heazlewood, S. Y., & Nilsson, S. K. (2019). Osteopontin is an important regulative component of the fetal bone marrow hematopoietic stem cell niche. Cells, 8(9), 985.
Boulais, P. E., & Frenette, P. S. (2015). Making sense of hematopoietic stem cell niches. Blood, 125(17), 2621–2629.
Montecino-Rodriguez, E., & Dorshkind, K. (2012). B-1 B cell development in the fetus and adult. Immunity, 36(1), 13–21.
Souto-Carneiro, M. M., Sims, G. P., Girschik, H., Lee, J., & Lipsky, P. E. (2005). Developmental changes in the human heavy chain CDR3. The Journal of Immunology, 175(11), 7425–7436.
Roy, A., Bystry, V., Bohn, G., Goudevenou, K., Reigl, T., Papaioannou, M., Krejci, A., O’Byrne, S., Chaidos, A., Grioni, A., et al. (2017). High resolution IgH repertoire analysis reveals fetal liver as the likely origin of life-long, innate B lymphopoiesis in humans. Clinical Immunology, 183, 8–16.
Gronwall, C., Vas, J., & Silverman, G. J. (2012). Protective roles of natural IgM antibodies. Frontiers in Immunology, 3, 66.
Shaw, P. X., Horkko, S., Chang, M. K., Curtiss, L. K., Palinski, W., Silverman, G. J., & Witztum, J. L. (2000). Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. The Journal of Clinical Investigation, 105(12), 1731–1740.
Moins-Teisserenc, H., Busson, M., Herda, A., & Apete, S. (2013). Peffault de Latour R, Robin M, Xhaard A, Toubert A, Socie G: CD19+CD5+ B cells and B1-like cells following allogeneic hematopoietic stem cell transplantation. Biology of Blood and Marrow Transplantation, 19(6), 988–991.
Xu, X., Deobagkar-Lele, M., Bull, K. R., Crockford, T. L., Mead, A. J., Cribbs, A. P., Sims, D., Anzilotti, C., & Cornall, R. J. (2020). An ontogenetic switch drives the positive and negative selection of B cells. Proceedings of the National Academy of Sciences U S A, 117(7), 3718–3727.
Yoshimoto, M., Montecino-Rodriguez, E., Ferkowicz, M. J., Porayette, P., Shelley, W. C., Conway, S. J., Dorshkind, K., & Yoder, M. C. (2011). Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proceedings of the National Academy of Sciences, 108(4), 1468–1473.
Yoshimoto, M. (2015). The first wave of B lymphopoiesis develops independently of stem cells in the murine embryo. Annals of the New York Academy of Sciences, 1362, 16–22.
Montecino-Rodriguez, E., Leathers, H., & Dorshkind, K. (2006). Identification of a B-1 B cell-specified progenitor. Nature Immunology, 7(3), 293–301.
Kobayashi, M., Shelley, W. C., Seo, W., Vemula, S., Lin, Y., Liu, Y., Kapur, R., Taniuchi, I., & Yoshimoto, M. (2014). Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfbeta for their development. Proceedings of the National Academy of Sciences U S A, 111(33), 12151–12156.
Vosshenrich, C. A., Cumano, A., Muller, W., Di Santo, J. P., & Vieira, P. (2004). Pre-B cell receptor expression is necessary for thymic stromal lymphopoietin responsiveness in the bone marrow but not in the liver environment. Proceedings of the National Academy of Sciences U S A, 101(30), 11070–11075.
Carpino, N., Thierfelder, W. E., Chang, M. S., Saris, C., Turner, S. J., Ziegler, S. F., & Ihle, J. N. (2004). Absence of an essential role for thymic stromal lymphopoietin receptor in murine B-cell development. Molecular and Cellular Biology, 24(6), 2584–2592.
Alhaj Hussen, K., Vu Manh, T. P., Guimiot, F., Nelson, E., Chabaane, E., Delord, M., Barbier, M., Berthault, C., Dulphy, N., Alberdi, A. J., et al. (2017). Molecular and functional characterization of lymphoid progenitor subsets reveals a bipartite architecture of human lymphopoiesis. Immunity, 47(4), 680-696 e688.
Sanz, E., Munoz, A. N., Monserrat, J., Van-Den-Rym, A., Escoll, P., Ranz, I., & Alvarez-Mon, M. (2010). de-la-Hera A: Ordering human CD34+CD10-CD19+ pre/pro-B-cell and CD19- common lymphoid progenitor stages in two pro-B-cell development pathways. Proceedings of the National Academy of Sciences U S A, 107(13), 5925–5930.
Sanz, E., Alvarez-Mon, M., Martinez, A. C., & de la Hera, A. (2003). Human cord blood CD34+Pax-5+B-cell progenitors: Single-cell analyses of their gene expression profiles. Blood, 101(9), 3424–3430.
O’Byrne, S., Elliott, N., Rice, S., Buck, G., Fordham, N., Garnett, C., Godfrey, L., Crump, N. T., Wright, G., Inglott, S., et al. (2019). Discovery of a CD10-negative B-progenitor in human fetal life identifies unique ontogeny-related developmental programs. Blood, 134(13), 1059–1071.
Boiers, C., Richardson, S. E., Laycock, E., Zriwil, A., Turati, V. A., Brown, J., Wray, J. P., Wang, D., James, C., Herrero, J., et al. (2018). A human IPS model implicates embryonic B-Myeloid fate restriction as developmental susceptibility to B acute lymphoblastic leukemia-associated ETV6-RUNX1. Developmental Cell, 44(3), 362–377367.
Roy, A., Cowan, G., Mead, A. J., Filippi, S., Bohn, G., Chaidos, A., Tunstall, O., Chan, J. K., Choolani, M., Bennett, P., et al. (2012). Perturbation of fetal liver hematopoietic stem and progenitor cell development by trisomy 21. Proceedings of the National Academy of Sciences U S A, 109(43), 17579–17584.
Michaelsson, J., Mold, J. E., McCune, J. M., & Nixon, D. F. (2006). Regulation of T cell responses in the developing human fetus. The Journal of Immunology, 176(10), 5741–5748.
Takahata, Y., Nomura, A., Takada, H., Ohga, S., Furuno, K., Hikino, S., Nakayama, H., Sakaguchi, S., & Hara, T. (2004). CD25+CD4+T cells in human cord blood: An immunoregulatory subset with naive phenotype and specific expression of forkhead box p3 (Foxp3) gene. Experimental Hematology, 32(7), 622–629.
Mold, J. E., Venkatasubrahmanyam, S., Burt, T. D., Michaelsson, J., Rivera, J. M., Galkina, S. A., Weinberg, K., Stoddart, C. A., & McCune, J. M. (2010). Fetal and adult hematopoietic stem cells give rise to distinct T cell lineages in humans. Science, 330(6011), 1695–1699.
Rudd, B. D. (2020). Neonatal T cells: A reinterpretation. Annual Review of Immunology, 38, 229–247.
Chea, S., Schmutz, S., Berthault, C., Perchet, T., Petit, M., Burlen-Defranoux, O., Goldrath, A. W., Rodewald, H. R., Cumano, A., & Golub, R. (2016). Single-cell gene expression analyses reveal heterogeneous responsiveness of fetal innate lymphoid progenitors to notch signaling. Cell reports, 14(6), 1500–1516.
Rackaityte, E., & Halkias, J. (2020). Mechanisms of fetal T cell tolerance and immune regulation. Frontiers in Immunology, 11, 588.
Xu, M. J., Matsuoka, S., Yang, F. C., Ebihara, Y., Manabe, A., Tanaka, R., Eguchi, M., Asano, S., Nakahata, T., & Tsuji, K. (2001). Evidence for the presence of murine primitive megakaryocytopoiesis in the early yolk sac. Blood, 97(7), 2016–2022.
Ma, D. C., Sun, Y. H., Chang, K. Z., & Zuo, W. (1996). Developmental change of megakaryocyte maturation and DNA ploidy in human fetus. European Journal of Haematology, 57(2), 121–127.
Sparger, K. A., Ramsey, H., Lorenz, V., Liu, Z. J., Feldman, H. A., Li, N., Laforest, T., & Sola-Visner, M. C. (2018). Developmental differences between newborn and adult mice in response to romiplostim. Platelets, 29(4), 365–372.
Israels, S. J., Odaibo, F. S., Robertson, C., McMillan, E. M., & McNicol, A. (1997). Deficient thromboxane synthesis and response in platelets from premature infants. Pediatric Research, 41(2), 218–223.
Davizon-Castillo, P., Allawzi, A., Sorrells, M., Fisher, S., Baltrunaite, K., Neeves, K., Nozik-Grayck, E., DiPaola, J., & Delaney, C. (2020). Platelet activation in experimental murine neonatal pulmonary hypertension. Physiological Reports, 8(5), 14386.
Margraf, A., Nussbaum, C., Rohwedder, I., Klapproth, S., Kurz, A. R. M., Florian, A., Wiebking, V., Pircher, J., Pruenster, M., Immler, R., et al. (2017). Maturation of platelet function during murine fetal development in vivo. Arteriosclerosis, Thrombosis, and Vascular Biology, 37(6), 1076–1086.
Stolla, M. C., Catherman, S. C., Kingsley, P. D., Rowe, R. G., Koniski, A. D., Fegan, K., Vit, L., McGrath, K. E., Daley, G. Q., & Palis, J. (2019). Lin28b regulates age-dependent differences in murine platelet function. Blood Advances, 3(1), 72–82.
Rowe, R. G., Wang, L. D., Coma, S., Han, A., Mathieu, R., Pearson, D. S., Ross, S., Sousa, P., Nguyen, P. T., Rodriguez, A., et al. (2016). Developmental regulation of myeloerythroid progenitor function by the Lin28b-let-7-Hmga2 axis. Journal of Experimental Medicine, 213(8), 1497–1512.
Dzierzak, E., & Philipsen, S. (2013). Erythropoiesis: Development and differentiation. Cold Spring Harbor Perspectives in Medicine, 3(4), 011601.
Linderkamp, O., Wu, P. Y., & Meiselman, H. J. (1983). Geometry of neonatal and adult red blood cells. Pediatric Research, 17(4), 250–253.
Kim, I., Saunders, T. L., & Morrison, S. J. (2007). Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell, 130(3), 470–483.
Oliveira-Mateos, C., Sanchez-Castillo, A., Soler, M., Obiols-Guardia, A., Pineyro, D., Boque-Sastre, R., Calleja-Cervantes, M. E., Castro de Moura, M., Martinez-Cardus, A., Rubio, T., et al. (2019). The transcribed pseudogene RPSAP52 enhances the oncofetal HMGA2-IGF2BP2-RAS axis through LIN28B-dependent and independent let-7 inhibition. Nature Communications, 10(1), 3979.
Li, Y. S., Zhou, Y., Tang, L., Shinton, S. A., Hayakawa, K., & Hardy, R. R. (2015). A developmental switch between fetal and adult B lymphopoiesis. Annals of the New York Academy of Sciences, 1362, 8–15.
McKinney-Freeman, S., Cahan, P., Li, H., Lacadie, S. A., Huang, H. T., Curran, M., Loewer, S., Naveiras, O., Kathrein, K. L., Konantz, M., et al. (2012). The transcriptional landscape of hematopoietic stem cell ontogeny. Cell Stem Cell, 11(5), 701–714.
Xu, J., Shao, Z., Glass, K., Bauer, D. E., Pinello, L., Van Handel, B., Hou, S., Stamatoyannopoulos, J. A., Mikkola, H. K., Yuan, G. C., et al. (2012). Combinatorial assembly of developmental stage-specific enhancers controls gene expression programs during human erythropoiesis. Developmental Cell, 23(4), 796–811.
Huang, J., Liu, X., Li, D., Shao, Z., Cao, H., Zhang, Y., Trompouki, E., Bowman, T. V., Zon, L. I., Yuan, G. C., et al. (2016). Dynamic Control of Enhancer Repertoires Drives Lineage and Stage-Specific Transcription during Hematopoiesis. Developmental Cell, 36(1), 9–23.
Chen, C., Yu, W., Tober, J., Gao, P., He, B., Lee, K., Trieu, T., Blobel, G. A., Speck, N. A., & Tan, K. (2019). Spatial genome re-organization between fetal and adult hematopoietic stem cells. Cell Reports, 29(12), 4200-4211 e4207.
Gomes, A. M., Kurochkin, I., Chang, B., Daniel, M., Law, K., Satija, N., Lachmann, A., Wang, Z., Ferreira, L., Ma’ayan, A., et al. (2018). Cooperative transcription factor induction mediates hemogenic reprogramming. Cell reports, 25(10), 2821-2835 e2827.
Lopez, C. K., Noguera, E., Stavropoulou, V., Robert, E., Aid, Z., Ballerini, P., Bilhou-Nabera, C., Lapillonne, H., Boudia, F., Thirant, C., et al. (2019). Ontogenic changes in hematopoietic hierarchy determine pediatric specificity and disease phenotype in fusion oncogene-driven myeloid leukemia. Cancer Discovery, 9(12), 1736–1753.
Hotfilder, M., Rottgers, S., Rosemann, A., Schrauder, A., Schrappe, M., Pieters, R., Jurgens, H., Harbott, J., & Vormoor, J. (2005). Leukemic stem cells in childhood high-risk ALL/t(9;22) and t(4;11) are present in primitive lymphoid-restricted CD34+CD19- cells. Cancer Research, 65(4), 1442–1449.
Ford, A. M., Bennett, C. A., Price, C. M., Bruin, M. C., Van Wering, E. R., & Greaves, M. (1998). Fetal origins of the TEL-AML1 fusion gene in identical twins with leukemia. Proceedings of the National Academy of Sciences, 95(8), 4584–4588.
Wiemels, J. L., Cazzaniga, G., Daniotti, M., Eden, O. B., Addison, G. M., Masera, G., Saha, V., Biondi, A., & Greaves, M. F. (1999). Prenatal origin of acute lymphoblastic leukaemia in children. Lancet, 354(9189), 1499–1503.
Zuna J, Madzo J, Krejci O, Zemanova Z, Kalinova M, Muzikova K, Zapotocky M, Starkova J, Hrusak O, Horak J et al (2011) ETV6/RUNX1 (TEL/AML1) is a frequent prenatal first hit in childhood leukemia. Blood, 117(1):368–369; author reply 370–361.
Lausten-Thomsen, U., Madsen, H. O., Vestergaard, T. R., Hjalgrim, H., Nersting, J., & Schmiegelow, K. (2011). Prevalence of t(12;21)[ETV6-RUNX1]-positive cells in healthy neonates. Blood, 117(1), 186–189.
Fueller, E., Schaefer, D., Fischer, U., Krell, P. F., Stanulla, M., Borkhardt, A., & Slany, R. K. (2014). Genomic inverse PCR for exploration of ligated breakpoints (GIPFEL), a new method to detect translocations in leukemia. PLoS ONE, 9(8), e104419.
Kosik, P., Skorvaga, M., Durdik, M., Jakl, L., Nikitina, E., Markova, E., Kozics, K., Horvathova, E., & Belyaev, I. (2017). Low numbers of pre-leukemic fusion genes are frequently present in umbilical cord blood without affecting DNA damage response. Oncotarget, 8(22), 35824–35834.
Skorvaga, M., Nikitina, E., Kubes, M., Kosik, P., Gajdosechova, B., Leitnerova, M., Copakova, L., & Belyaev, I. (2014). Incidence of common preleukemic gene fusions in umbilical cord blood in Slovak population. PLoS ONE, 9(3), e91116.
Schafer, D., Olsen, M., Lahnemann, D., Stanulla, M., Slany, R., Schmiegelow, K., Borkhardt, A., & Fischer, U. (2018). Five percent of healthy newborns have an ETV6-RUNX1 fusion as revealed by DNA-based GIPFEL screening. Blood, 131(7), 821–826.
Hein, D., Dreisig, K., Metzler, M., Izraeli, S., Schmiegelow, K., Borkhardt, A., & Fischer, U. (2019). The preleukemic TCF3-PBX1 gene fusion can be generated in utero and is present in approximately 0.6% of healthy newborns. Blood, 134(16), 1355–1358.
Puumala, S. E., Ross, J. A., Aplenc, R., & Spector, L. G. (2013). Epidemiology of childhood acute myeloid leukemia. Pediatric Blood and Cancer, 60(5), 728–733.
Andersson, A. K., Ma, J., Wang, J., Chen, X., Gedman, A. L., Dang, J., Nakitandwe, J., Holmfeldt, L., Parker, M., Easton, J., et al. (2015). The landscape of somatic mutations in infant MLL-rearranged acute lymphoblastic leukemias. Nature Genetics, 47(4), 330–337.
Pine, S. R., Guo, Q., Yin, C., Jayabose, S., Druschel, C. M., & Sandoval, C. (2007). Incidence and clinical implications of GATA1 mutations in newborns with Down syndrome. Blood, 110(6), 2128–2131.
Horton, S. J., Jaques, J., Woolthuis, C., van Dijk, J., Mesuraca, M., Huls, G., Morrone, G., Vellenga, E., & Schuringa, J. J. (2013). MLL-AF9-mediated immortalization of human hematopoietic cells along different lineages changes during ontogeny. Leukemia, 27(5), 1116–1126.
Liang, D. C., Liu, H. C., Yang, C. P., Jaing, T. H., Hung, I. J., Yeh, T. C., Chen, S. H., Hou, J. Y., Huang, Y. J., Shih, Y. S., et al. (2013). Cooperating gene mutations in childhood acute myeloid leukemia with special reference on mutations of ASXL1, TET2, IDH1, IDH2, and DNMT3A. Blood, 121(15), 2988–2995.
Cancer Genome Atlas Research N (2013) Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. New England Journal of Medicine, 368(22): 2059–2074.
Bateman, C. M., Colman, S. M., Chaplin, T., Young, B. D., Eden, T. O., Bhakta, M., Gratias, E. J., van Wering, E. R., Cazzaniga, G., Harrison, C. J., et al. (2010). Acquisition of genome-wide copy number alterations in monozygotic twins with acute lymphoblastic leukemia. Blood, 115(17), 3553–3558.
Pagano, L., Pulsoni, A., Vignetti, M., Mele, L., Fianchi, L., Petti, M. C., Mirto, S., Falcucci, P., Fazi, P., Broccia, G., et al. (2002). Acute megakaryoblastic leukemia: Experience of GIMEMA trials. Leukemia, 16(9), 1622–1626.
Antonarakis, S. E., Lyle, R., Dermitzakis, E. T., Reymond, A., & Deutsch, S. (2004). Chromosome 21 and down syndrome: From genomics to pathophysiology. Nature Reviews Genetics, 5(10), 725–738.
Henry, E., Walker, D., Wiedmeier, S. E., & Christensen, R. D. (2007). Hematological abnormalities during the first week of life among neonates with Down syndrome: Data from a multihospital healthcare system. American Journal of Medical Genetics. Part A, 143A(1), 42–50.
de Rooij, J. D., Branstetter, C., Ma, J., Li, Y., Walsh, M. P., Cheng, J., Obulkasim, A., Dang, J., Easton, J., Verboon, L. J., et al. (2017). Pediatric non-down syndrome acute megakaryoblastic leukemia is characterized by distinct genomic subsets with varying outcomes. Nature Genetics, 49(3), 451–456.
Gruber, T. A., & Downing, J. R. (2015). The biology of pediatric acute megakaryoblastic leukemia. Blood, 126(8), 943–949.
Gruber, T. A., Larson Gedman, A., Zhang, J., Koss, C. S., Marada, S., Ta, H. Q., Chen, S. C., Su, X., Ogden, S. K., Dang, J., et al. (2012). An Inv(16)(p13.3q24.3)-encoded CBFA2T3-GLIS2 fusion protein defines an aggressive subtype of pediatric acute megakaryoblastic leukemia. Cancer Cell, 22(5), 683–697.
Morrow, M., Horton, S., Kioussis, D., Brady, H. J., & Williams, O. (2004). TEL-AML1 promotes development of specific hematopoietic lineages consistent with preleukemic activity. Blood, 103(10), 3890–3896.
Torrano, V., Procter, J., Cardus, P., Greaves, M., & Ford, A. M. (2011). ETV6-RUNX1 promotes survival of early B lineage progenitor cells via a dysregulated erythropoietin receptor. Blood, 118(18), 4910–4918.
Martin-Lorenzo, A., Hauer, J., Vicente-Duenas, C., Auer, F., Gonzalez-Herrero, I., Garcia-Ramirez, I., Ginzel, S., Thiele, R., Constantinescu, S. N., Bartenhagen, C., et al. (2015). Infection exposure is a causal factor in B-cell precursor acute lymphoblastic leukemia as a result of Pax5-Inherited susceptibility. Cancer Discovery, 5(12), 1328–1343.
Rodriguez-Hernandez, G., Hauer, J., Martin-Lorenzo, A., Schafer, D., Bartenhagen, C., Garcia-Ramirez, I., Auer, F., Gonzalez-Herrero, I., Ruiz-Roca, L., Gombert, M., et al. (2017). Infection Exposure Promotes ETV6-RUNX1 Precursor B-cell Leukemia via Impaired H3K4 Demethylases. Cancer Research, 77(16), 4365–4377.
Burgler, S., & Nadal, D. (2017). Pediatric precursor B acute lymphoblastic leukemia: Are T helper cells the missing link in the infectious etiology theory? Molecular and Cellular Pediatrics, 4(1), 6.
Sullivan, E. M., Jeha, S., Kang, G., Cheng, C., Rooney, B., Holladay, M., Bari, R., Schell, S., Tuggle, M., Pui, C. H., et al. (2014). NK cell genotype and phenotype at diagnosis of acute lymphoblastic leukemia correlate with postinduction residual disease. Clinical Cancer Research, 20(23), 5986–5994.
Cazzaniga, G., van Delft, F. W., Lo Nigro, L., Ford, A. M., Score, J., Iacobucci, I., Mirabile, E., Taj, M., Colman, S. M., Biondi, A., et al. (2011). Developmental origins and impact of BCR-ABL1 fusion and IKZF1 deletions in monozygotic twins with Ph+ acute lymphoblastic leukemia. Blood, 118(20), 5559–5564.
Wiemels, J. L., Ford, A. M., Van Wering, E. R., Postma, A., & Greaves, M. (1999). Protracted and variable latency of acute lymphoblastic leukemia after TEL-AML1 gene fusion in utero. Blood, 94(3), 1057–1062.
Maia, A. T., van der Velden, V. H., Harrison, C. J., Szczepanski, T., Williams, M. D., Griffiths, M. J., van Dongen, J. J., & Greaves, M. F. (2003). Prenatal origin of hyperdiploid acute lymphoblastic leukemia in identical twins. Leukemia, 17(11), 2202–2206.
Gamis, A. S., & Smith, F. O. (2012). Transient myeloproliferative disorder in children with Down syndrome: Clarity to this enigmatic disorder. British Journal of Haematology, 159(3), 277–287.
Matsuda, K., Shimada, A., Yoshida, N., Ogawa, A., Watanabe, A., Yajima, S., Iizuka, S., Koike, K., Yanai, F., Kawasaki, K., et al. (2007). Spontaneous improvement of hematologic abnormalities in patients having juvenile myelomonocytic leukemia with specific RAS mutations. Blood, 109(12), 5477–5480.
Pieters, R., De Lorenzo, P., Ancliffe, P., Aversa, L. A., Brethon, B., Biondi, A., Campbell, M., Escherich, G., Ferster, A., Gardner, R. A., et al. (2019). Outcome of infants younger than 1 year with acute lymphoblastic leukemia treated with the interfant-06 protocol: Results from an international phase III randomized study. Journal of Clinical Oncology, 37(25), 2246–2256.
Tomizawa, D., Miyamura, T., Imamura, T., Watanabe, T., Moriya Saito, A., Ogawa, A., Takahashi, Y., Hirayama, M., Taki, T., Deguchi, T., et al. (2020). A risk-stratified therapy for infants with acute lymphoblastic leukemia: A report from the JPLSG MLL-10 trial. Blood, 136(16), 1813–1823.
Pui, C. H., Carroll, W. L., Meshinchi, S., & Arceci, R. J. (2011). Biology, risk stratification, and therapy of pediatric acute leukemias: An update. Journal of Clinical Oncology, 29(5), 551–565.
Salzer, W. L., Devidas, M., Carroll, W. L., Winick, N., Pullen, J., Hunger, S. P., & Camitta, B. A. (2010). Long-term results of the pediatric oncology group studies for childhood acute lymphoblastic leukemia 1984–2001: A report from the children’s oncology group. Leukemia, 24(2), 355–370.
Jackson, T. R., Ling, R. E., & Roy, A. (2021). The Origin of B-cells: Human Fetal B Cell development and implications for the pathogenesis of childhood acute lymphoblastic leukemia. Frontiers in immunology, 12, 637975.
Greaves, M. (2018). A causal mechanism for childhood acute lymphoblastic leukaemia. Nature Reviews Cancer, 18(8), 471–484.
Montecino-Rodriguez, E., Li, K., Fice, M., & Dorshkind, K. (2014). Murine B-1 B cell progenitors initiate B-acute lymphoblastic leukemia with features of high-risk disease. The Journal of Immunology, 192(11), 5171–5178.
Choi, Y. J., Heck, A. M., Hayes, B. J., Lih, D., Rayner, S. G., Hadland, B., & Zheng, Y. (2021). WNT5A from the fetal liver vascular niche supports human fetal liver hematopoiesis. Stem Cell Research and Therapy, 12(1), 321.
Funding
This work was supported by NIH grants R01 HL133560-01 and R01 CA223194-01 through Loyola University Chicago, as well as Loyola program development funds to Jiwang Zhang.
Author information
Authors and Affiliations
Contributions
Ryan Mack and Lei Zhang drafted the first version of this review. All authors contributed to the writing of this manuscript. Peter Breslin did the final editing. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of Interest
The authors declare that they have no competing financial or professional interests.
Ethics Approval
This is not applicable for this review.
Consent to Participate
This is not applicable for this review.
Consent for Publication
This is not applicable for this review.
Availability of Data and Material
This is not applicable for this review.
Code Availability
This is not applicable for this review.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mack, R., Zhang, L., Breslin, SJ, P. et al. The Fetal-to-Adult Hematopoietic Stem Cell Transition and its Role in Childhood Hematopoietic Malignancies. Stem Cell Rev and Rep 17, 2059–2080 (2021). https://doi.org/10.1007/s12015-021-10230-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10230-x