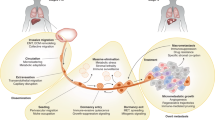
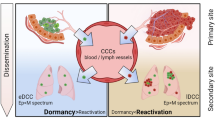
Abstract
Tumor invasion and metastasis remain the leading causes of mortality for patients with cancer despite current treatment strategies. In some cancer types, recurrence is considered inevitable due to the lack of effective anti-metastatic therapies. Recent studies across many cancer types demonstrate a close relationship between cancer-initiating cells (CICs) and metastasis, as well as general cancer progression. First, this review describes CICs’ contribution to cancer progression. Then we discuss our recent understanding of mechanisms through which CICs promote tumor invasion and metastasis by examining the role of CICs in each stage. Finally, we examine the current understanding of CICs’ contribution to therapeutic resistance and recent developments in CIC-targeting drugs. We believe this understanding is key to advancing anti-CIC clinical therapeutics.
Graphical Abstract



Similar content being viewed by others
References
Zakrzewski, W., et al. (2019). Stem cells: Past, present, and future. Stem cell Research & Therapy, 10(1), 1–22.
Lau, K. X., et al. (2020). Unique properties of a subset of human pluripotent stem cells with high capacity for self-renewal. Nature Communications, 11(1), 1–18.
Tu, S.-M., Lin, S.-H., & Logothetis, C. J. (2002). Stem-cell origin of metastasis and heterogeneity in solid tumours. The Lancet Oncology, 3(8), 508–513.
Guo, L., et al. (2011). Selection of brain metastasis-initiating breast cancer cells determined by growth on hard agar. The American Journal of Pathology, 178(5), 2357–2366.
Li, S., & Li, Q. (2014). Cancer stem cells and tumor metastasis. International Journal of Oncology, 44(6), 1806–1812.
Shiozawa, Y., et al. (2013). Cancer stem cells and their role in metastasis. Pharmacology & Therapeutics, 138(2), 285–293.
Wagers, A. J., & Weissman, I. L. (2004). Plasticity of adult stem cells. Cell, 116(5), 639–648.
Reya, T., et al. (2001). Stem cells, cancer, and cancer stem cells. Nature, 414(6859), 105–111.
Lytle, N. K., Barber, A. G., & Reya, T. (2018). Stem cell fate in cancer growth, progression and therapy resistance. Nature Reviews Cancer, 18(11), 669–680.
Kimbrel, E. A., & Lanza, R. (2020). Next-generation stem cells—ushering in a new era of cell-based therapies. Nature Reviews Drug Discovery, 9, 463–479. https://doi.org/10.1038/s41573-020-0064-x
Ermolaeva, M., et al. (2018). Cellular and epigenetic drivers of stem cell ageing. Nature Reviews Molecular Cell Biology, 19(9), 594.
Becker, A. J., McCulloch, E. A., & Till, J. E. (1963). Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature, 197(4866), 452–454.
Evans, M. J., & Kaufman, M. H. (1981). Establishment in culture of pluripotential cells from mouse embryos. Nature, 292(5819), 154–156.
Martin, G. R. (1981). Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proceedings of the National Academy of Sciences, 78(12), 7634–7638.
Thomson, J. A., et al. (1998). Embryonic stem cell lines derived from human blastocysts. Science, 282(5391), 1145–1147.
Watt, F. M., & Driskell, R. R. (2010). The therapeutic potential of stem cells. Philosophical Transactions of the Royal Society B: Biological Sciences, 365(1537), 155–163.
Takahashi, K., & Yamanaka, S. (2013). Induced pluripotent stem cells in medicine and biology. Development, 140(12), 2457–2461.
Shen, Q., et al. (2004). Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science, 304(5675), 1338–1340.
Wang, Y. X., Dumont, N. A., & Rudnicki, M. A. (2014). Muscle stem cells at a glance. The Company of Biologists Ltd.
Fukada, S. I., et al. (2007). Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells, 25(10), 2448–2459.
Gnocchi, V. F., et al. (2009). Further characterisation of the molecular signature of quiescent and activated mouse muscle satellite cells. PLoS One, 4(4), e5205.
Ng, A., & Barker, N. (2015). Ovary and fimbrial stem cells: Biology, niche and cancer origins. Nature Reviews Molecular Cell Biology, 16(10), 625–638.
Barker, N., et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature, 449(7165), 1003–1007.
Park, D., Sykes, D. B., & Scadden, D. T. (2012). The hematopoietic stem cell niche. Frontiers in Bioscience (Landmark Edition), 17, 30.
Garg, S., Madkaikar, M., & Ghosh, K. (2013). Investigating cell surface markers on normal hematopoietic stem cells in three different niche conditions. International Journal of Stem Cells, 6(2), 129.
Morrison, S. J., & Scadden, D. T. (2014). The bone marrow niche for haematopoietic stem cells. Nature, 505(7483), 327–334.
Morrison, S. J., & Kimble, J. (2006). Asymmetric and symmetric stem-cell divisions in development and cancer. Nature, 441(7097), 1068–1074.
Fuchs, E., & Chen, T. (2013). A matter of life and death: Self-renewal in stem cells. EMBO Reports, 14(1), 39–48.
Jan, Y.-N., & Jan, L. Y. (2000). Polarity in cell division: What frames thy fearful asymmetry? Cell, 100(6), 599–602.
Ito, K., & Suda, T. (2014). Metabolic requirements for the maintenance of self-renewing stem cells. Nature Reviews Molecular Cell Biology, 15(4), 243–256.
Neumüller, R. A., & Knoblich, J. A. (2009). Dividing cellular asymmetry: Asymmetric cell division and its implications for stem cells and cancer. Genes & Development, 23(23), 2675–2699.
Shlyakhtina, Y., Moran, K. L., & Portal, M. M. (2019). Asymmetric inheritance of cell fate determinants: Focus on RNA. Non-Coding RNA, 5(2), 38.
Betschinger, J., Mechtler, K., & Knoblich, J. A. (2003). The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature, 422(6929), 326–330.
Morin, X., & Bellaïche, Y. (2011). Mitotic spindle orientation in asymmetric and symmetric cell divisions during animal development. Developmental Cell, 21(1), 102–119.
Spradling, A., Drummond-Barbosa, D., & Kai, T. (2001). Stem cells find their niche. Nature, 414(6859), 98–104.
Bigas, A., & Espinosa, L. (2012). Hematopoietic stem cells: To be or Notch to be. Blood, 119(14), 3226–3235.
Wilson, A., & Radtke, F. (2006). Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Letters, 580(12), 2860–2868.
Jia, Y., Wang, Y., & Xie, J. (2015). The Hedgehog pathway: Role in cell differentiation, polarity and proliferation. Archives of Toxicology, 89(2), 179–191.
Oliver, T. G., et al. (2003). Transcriptional profiling of the Sonic hedgehog response: A critical role for N-myc in proliferation of neuronal precursors. Proceedings of the National Academy of Sciences, 100(12), 7331–7336.
Jung, Y.-S., & Park, J.-I. (2020). Wnt signaling in cancer: therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Experimental & Molecular Medicine, 52, 183–191. https://doi.org/10.1038/s12276-020-0380-6
Levy, V., et al. (2005). Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Developmental Cell, 9(6), 855–861.
Selman, K., & Kafatos, F. C. (1974). Transdifferentiation in the labial gland of silk moths: Is DNA required for cellular metamorphosis? Cell Differentiation, 3(2), 81–94.
Slack, J. (1986). Epithelial metaplasia and the second anatomy. The Lancet, 328(8501), 268–271.
Christoforou, N., et al. (2017). Core transcription factors, microRNAs, and small molecules drive transdifferentiation of human fibroblasts towards the cardiac cell lineage. Scientific Reports, 7(1), 1–15.
Camargo, F. D., et al. (2003). Single hematopoietic stem cells generate skeletal muscle through myeloid intermediates. Nature Medicine, 9(12), 1520–1527.
Aghajanian, P., & Mohan, S. (2018). The art of building bone: Emerging role of chondrocyte-to-osteoblast transdifferentiation in endochondral ossification. Bone Research, 6(1), 1–9.
Slack, J. M. (2007). Metaplasia and transdifferentiation: From pure biology to the clinic. Nature Reviews Molecular Cell biology, 8(5), 369–378.
Slack, J. (1985). Homoeotic transformations in man: Implications for the mechanism of embryonic development and for the organization of epithelia. Journal of Theoretical Biology, 114(3), 463.
Mollinari, C., et al. (2018). Transdifferentiation: A new promise for neurodegenerative diseases. Cell Death & Disease, 9(8), 1–9.
Silberg, D. G., et al. (2002). Cdx2 ectopic expression induces gastric intestinal metaplasia in transgenic mice. Gastroenterology, 122(3), 689–696.
Treviño, L. S., et al. (2020). Epigenome environment interactions accelerate epigenomic aging and unlock metabolically restricted epigenetic reprogramming in adulthood. Nature Communications, 11(1), 1–14.
Wahl, G. M., & Spike, B. T. (2017). Cell state plasticity, stem cells, EMT, and the generation of intra-tumoral heterogeneity. NPJ Breast Cancer, 3(1), 1–13.
Greaves, M., & Maley, C. C. (2012). Clonal evolution in cancer. Nature, 481(7381), 306–313.
O’Flaherty, J. D., et al. (2012). The cancer stem-cell hypothesis: Its emerging role in lung cancer biology and its relevance for future therapy. Journal of Thoracic Oncology, 7(12), 1880–1890.
Borah, A., et al. (2015). Targeting self-renewal pathways in cancer stem cells: Clinical implications for cancer therapy. Oncogenesis, 4(11), e177–e177.
Brabletz, T., et al. (2005). Migrating cancer stem cells—an integrated concept of malignant tumour progression. Nature Reviews Cancer, 5(9), 744–749.
Moharil, R. B., et al. (2017). Cancer stem cells: An insight. Journal of Oral and Maxillofacial Pathology: JOMFP, 21(3), 463.
Zhou, B.-B.S., et al. (2009). Tumour-initiating cells: Challenges and opportunities for anticancer drug discovery. Nature Reviews Drug Discovery, 8(10), 806–823.
Yu, Z., et al. (2012). Cancer Stem Cells. The international journal of biochemistry & cell biology, 44(12), 2144–2151.
Qureshi-Baig, K., et al. (2017). Tumor-initiating cells: A criTICal review of isolation approaches and new challenges in targeting strategies. Molecular Cancer, 16(1), 40.
Visvader, J. E. (2011). Cells of origin in cancer. Nature, 469(7330), 314–322.
Shekhani, M. T., et al. (2013). Cancer stem cells and tumor transdifferentiation: Implications for novel therapeutic strategies. American Journal of Stem Cells, 2(1), 52.
Friedmann-Morvinski, D., & Verma, I. M. (2014). Dedifferentiation and reprogramming: Origins of cancer stem cells. EMBO Reports, 15(3), 244–253.
Yang, L., et al. (2020). Targeting cancer stem cell pathways for cancer therapy. Signal Transduction and Targeted Therapy, 5(1), 1–35.
Bussolati, B., et al. (2009). Endothelial cell differentiation of human breast tumour stem/progenitor cells. Journal of Cellular and Molecular Medicine, 13(2), 309–319.
Uppal, A., et al. (2014). Investigation of the essential role of platelet-tumor cell interactions in metastasis progression using an agent-based model. Theoretical Biology and Medical Modelling, 11(1), 1–23.
Bielenberg, D. R., & Zetter, B. R. (2015). The contribution of angiogenesis to the process of metastasis. Cancer Journal (Sudbury, Mass.), 21(4), 267.
Zeidman, I. (1957). Metastasis: A review of recent advances. Cancer Research, 17(3), 157–162.
Balic, M., et al. (2006). Most early disseminated cancer cells detected in bone marrow of breast cancer patients have a putative breast cancer stem cell phenotype. Clinical Cancer Research, 12(19), 5615–5621.
Lucarelli, G., et al. (2015). Isolation and characterization of cancer stem cells in renal cell carcinoma. Urologia Journal, 82(1), 46–53.
Xie, L., et al. (2014). Isolation and identification of cancer stem cells from human LACC cell line. [Zhonghua yan ke za zhi] Chinese Journal of Ophthalmology, 50(10), 753–757.
Song, E. (2010). Research progress of solid tumor stem cells. Journal of Sun Yat-sen University (Medical Sciences), 31(2), 172–178.
Liu, W.-H., et al. (2012). Interpretation of interlocking key issues of cancer stem cells in malignant solid tumors. Cellular Oncology, 35(6), 397–409.
Wagenblast, E., et al. (2015). A model of breast cancer heterogeneity reveals vascular mimicry as a driver of metastasis. Nature, 520(7547), 358–362.
Castellón, E. A., et al. (2012). Molecular signature of cancer stem cells isolated from prostate carcinoma and expression of stem markers in different Gleason grades and metastasis. Biological Research, 45(3), 297–305.
Chen, S., Principessa, L., & Isaacs, J. T. (2012). Human prostate cancer initiating cells isolated directly from localized cancer do not form prostaspheres in primary culture. The Prostate, 72(13), 1478–1489.
Hou, J.-M., et al. (2012). Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. Journal of Clinical Oncology, 30(5), 525–532.
Zakikhani, M., et al. (2010). Metformin and rapamycin have distinct effects on the AKT pathway and proliferation in breast cancer cells. Breast Cancer Research and Treatment, 123(1), 271–279.
Morel, A.-P., et al. (2008). Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS One, 3(8), e2888.
El Hallani, S., et al. (2010). A new alternative mechanism in glioblastoma vascularization: Tubular vasculogenic mimicry. Brain, 133(4), 973–982.
Shen, R., et al. (2008). Precancerous stem cells can serve as tumor vasculogenic progenitors. PLoS One, 3(2), e1652.
Mathonnet, M., et al. (2014). Hallmarks in colorectal cancer: Angiogenesis and cancer stem-like cells. World Journal of Gastroenterology: WJG, 20(15), 4189.
Atzori, M. G., et al. (2017). The anti-vascular endothelial growth factor receptor-1 monoclonal antibody D16F7 inhibits invasiveness of human glioblastoma and glioblastoma stem cells. Journal of Experimental & Clinical Cancer Research, 36(1), 1–15.
Wieland, E., et al. (2017). Endothelial Notch1 activity facilitates metastasis. Cancer Cell, 31(3), 355–367.
Rosner, M., et al. (2017). Human stem cells alter the invasive properties of somatic cells via paracrine activation of mTORC1. Nature Communications, 8(1), 1–16.
You, N., et al. (2017). Tg737 acts as a key driver of invasion and migration in liver cancer stem cells and correlates with poor prognosis in patients with hepatocellular carcinoma. Experimental Cell Research, 358(2), 217–226.
Cao, W., et al. (2020). LGR5 marks targetable tumor-initiating cells in mouse liver cancer. Nature Communications, 11(1), 1–16.
Haas, T. L., et al. (2017). Integrin α7 is a functional marker and potential therapeutic target in glioblastoma. Cell Stem Cell, 21(1), 35-50.e9.
Avril, T., et al. (2017). CD90 expression controls migration and predicts dasatinib response in glioblastoma. Clinical Cancer Research, 23(23), 7360–7374.
Shibue, T., & Weinberg, R. A. (2017). EMT, CSCs, and drug resistance: The mechanistic link and clinical implications. Nature Reviews Clinical oncology, 14(10), 611.
Winkler, F. (2015). The brain metastatic niche. Journal of Molecular Medicine, 93(11), 1213–1220.
Liao, J., et al. (2014). Ovarian cancer spheroid cells with stem cell-like properties contribute to tumor generation, metastasis and chemotherapy resistance through hypoxia-resistant metabolism. PLoS One, 9(1), e84941.
Fong, M. Y., et al. (2015). Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nature Cell Biology, 17(2), 183–194.
Li, G., et al. (2010). CD133+ single cell-derived progenies of colorectal cancer cell line SW480 with different invasive and metastatic potential. Clinical & Experimental Metastasis, 27(7), 517–527.
Chinn, S. B., et al. (2015). Cancer stem cells: Mediators of tumorigenesis and metastasis in head and neck squamous cell carcinoma. Head & Neck, 37(3), 317–326.
Ayob, A. Z., & Ramasamy, T. S. (2018). Cancer stem cells as key drivers of tumour progression. Journal of Biomedical Science, 25(1), 1–18.
Moreno-Bueno, G., Portillo, F., & Cano, A. (2008). Transcriptional regulation of cell polarity in EMT and cancer. Oncogene, 27(55), 6958–6969.
Zhou, W., et al. (2014). Cancer-secreted miR-105 destroys vascular endothelial barriers to promote metastasis. Cancer Cell, 25(4), 501–515.
Grange, C., et al. (2011). Microvesicles released from human renal cancer stem cells stimulate angiogenesis and formation of lung premetastatic niche. Cancer Research, 71(15), 5346–5356.
Desurmont, T., et al. (2015). Overexpression of chemokine receptor CXCR 2 and ligand CXCL 7 in liver metastases from colon cancer is correlated to shorter disease-free and overall survival. Cancer Science, 106(3), 262–269.
Du, T., et al. (2014). Microvesicles derived from human Wharton’s jelly mesenchymal stem cells promote human renal cancer cell growth and aggressiveness through induction of hepatocyte growth factor. PLoS One, 9(5), e96836.
Venkatesh, V., et al. (2018). Targeting notch signalling pathway of cancer stem cells. Stem Cell Investigation, 5, 5.
Aster, J. C., Pear, W. S., & Blacklow, S. C. (2017). The varied roles of notch in cancer. Annual Review of Pathology: Mechanisms of Disease, 12, 245–275.
Gupta, S., Takebe, N., & LoRusso, P. (2010). Targeting the Hedgehog pathway in cancer. Therapeutic Advances in Medical Oncology, 2(4), 237–250.
Ning, X., et al. (2013). Therapeutic strategies targeting cancer stem cells. Cancer Biology & Therapy, 14(4), 295–303.
King, T., & Posey, A. (2019). Co-expression of an engineered cell-surface sialidase by CART cells improves anti-cancer activity of NK cells in solid tumors. Cytotherapy, 21(5), S27.
Wing, A., et al. (2018). Improving CART-cell therapy of solid tumors with oncolytic virus–driven production of a bispecific T-cell engager. Cancer Immunology Research, 6(5), 605–616.
Hervault, A., & Thanh, N. T. K. (2014). Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale, 6(20), 11553–11573.
Zhuang, Y., & Miskimins, W. (2008). Cell cycle arrest in Metformin treated breast cancer cells involves activation of AMPK, downregulation of cyclin D1, and requires p27 Kip1 or p21 Cip1. Journal of Molecular Signaling, 3(1), 18.
Hirsch, H. A., et al. (2009). Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Research, 69(19), 7507–7511.
Phoenix, K. N., Vumbaca, F., & Claffey, K. P. (2009). Therapeutic metformin/AMPK activation promotes the angiogenic phenotype in the ERα negative MDA-MB-435 breast cancer model. Breast Cancer Research and Treatment, 113(1), 101–111.
Seyfried, T. N., & Huysentruyt, L. C. (2013). On the origin of cancer metastasis. Critical Reviews in Oncogenesis, 18(1–2), 43.
Riccioni, R., et al. (2010). The cancer stem cell selective inhibitor salinomycin is a p-glycoprotein inhibitor. Blood Cells, Molecules, and Diseases, 45(1), 86–92.
Naujokat, C., & Steinhart, R. (2012). Salinomycin as a drug for targeting human cancer stem cells. BioMed Research International, 2012, 950658. https://doi.org/10.1155/2012/950658
Mai, T. T., et al. (2017). Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nature Chemistry, 9(10), 1025.
Wei, J., Sun, J., & Liu, Y. (2019). Enhanced targeting of prostate cancer-initiating cells by salinomycin-encapsulated lipid-PLGA nanoparticles linked with CD44 antibodies. Oncology Letters, 17(4), 4024–4033.
Wang, Y., et al. (2014). Roles of histamine on the expression of aldehyde dehydrogenase 1 in endometrioid adenocarcinoma cell line. Cancer medicine, 3(5), 1126–1135.
Duan, L., et al. (2014). Inhibitory effect of Disulfiram/copper complex on non-small cell lung cancer cells. Biochemical and Biophysical Research Communications, 446(4), 1010–1016.
Schech, A., et al. (2015). Histone deacetylase inhibitor entinostat inhibits tumor-initiating cells in triple-negative breast cancer cells. Molecular Cancer Therapeutics, 14(8), 1848–1857.
Shidal, C., et al. (2016). Lunasin is a novel therapeutic agent for targeting melanoma cancer stem cells. Oncotarget, 7(51), 84128.
Patel, N. S., et al. (2005). Up-regulation of delta-like 4 ligand in human tumor vasculature and the role of basal expression in endothelial cell function. Cancer Research, 65(19), 8690–8697.
Milas, L., & Hittelman, W. N. (2009). Cancer stem cells and tumor response to therapy: current problems and future prospects. Seminars in Radiation Oncology, 19(2), 96–105. https://doi.org/10.1016/j.semradonc.2008.11.004
Ma, H., Li, H.-Q., & Zhang, X. (2013). Cyclopamine, a naturally occurring alkaloid, and its analogues may find wide applications in cancer therapy. Current Topics in Medicinal Chemistry, 13(17), 2208–2215.
Chen, J. K. (2016). I only have eye for ewe: The discovery of cyclopamine and development of Hedgehog pathway-targeting drugs. Natural Product Reports, 33(5), 595–601.
Frampton, J. E., & Basset-Séguin, N. (2018). Vismodegib: A review in advanced basal cell carcinoma. Drugs, 78(11), 1145–1156.
Sharpe, H. J., et al. (2015). Genomic analysis of smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell, 27(3), 327–341.
Li, Q.-R., et al. (2019). Novel-smoothened inhibitors for therapeutic targeting of naïve and drug-resistant hedgehog pathway-driven cancers. Acta Pharmacologica Sinica, 40(2), 257–267.
Vincent, K. M., & Postovit, L.-M. (2017). A pan-cancer analysis of secreted Frizzled-related proteins: Re-examining their proposed tumour suppressive function. Scientific Reports, 7, 42719.
Ushijima, H., et al. (2015). Radio-sensitization effect of an mTOR inhibitor, temsirolimus, on lung adenocarcinoma A549 cells under normoxic and hypoxic conditions. Journal of Radiation Research, 56(4), 663–668.
Sun, J. D., et al. (2015). Combination treatment with hypoxia-activated prodrug evofosfamide (TH-302) and mTOR inhibitors results in enhanced antitumor efficacy in preclinical renal cell carcinoma models. American Journal of Cancer Research, 5(7), 2139.
Souza, D. G., et al. (2004). Repertaxin, a novel inhibitor of rat CXCR2 function, inhibits inflammatory responses that follow intestinal ischaemia and reperfusion injury. British Journal of Pharmacology, 143(1), 132–142.
Acknowledgements
This work was supported by the National Institute of Neurological Disorders and Stroke grant 1R01NS096376, 1R01NS112856 the American Cancer Society grant RSG-16-034-01-DDC (to A.U.A.) and P50CA221747 SPORE for Translational Approaches to Brain Cancer.
Author information
Authors and Affiliations
Contributions
Literature Review: Shivani Baisiwala, Shreya Budhiraja, Miranda Saathoff, Chirag Goel, Khizar Nandoliya.
Writing: Shivani Baisiwala, Shreya Budhiraja, Miranda Saathoff, Chirag Goel, Khizar Nandoliya.
Resources: Atique U Ahmed.
Editing: Shivani Baisiwala, Khizar Nandoliya, Atique U Ahmed.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Baisiwala, S., Budhiraja, S., Goel, C. et al. Spelling Out CICs: A Multi-Organ Examination of the Contributions of Cancer Initiating Cells’ Role in Tumor Progression. Stem Cell Rev and Rep 18, 228–240 (2022). https://doi.org/10.1007/s12015-021-10195-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10195-x