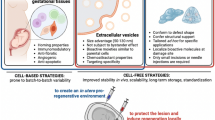
Abstract
Neural tube defects (NTDs) are among the most common congenital defects during neurulation. Spina bifida is a type of NTD that can occur in different forms. Since myelomeningocele (MMC) is the most severe form of spina bifida, finding a satisfactory treatment for MMC is a gold standard for the treatment of spina bifida. The Management of Myelomeningocele Study (MOMS) demonstrated that intrauterine treatment of spina bifida could ameliorate the complications associated with spina bifida and would also reduce the placement of ventriculoperitoneal (VP) shunt by 50%. Recently developed tissue engineering (TE) approaches using scaffolds, stem cells, and growth factors allow treatment of the fetus with minimally invasive methods and promising outcomes. The application of novel patches with appropriate stem cells and growth factors leads to better coverage of the defect with fewer complications. These approaches with less invasive surgical procedures, even in animal models with similar characteristics as the human MMC defect, paves the way for the modern application of less invasive surgical methods. Significantly, the early detection of these problems and applying these approaches can increase the potential efficacy of MMC treatment with fewer complications. However, further studies should be conducted to find the most suitable scaffolds and stem cells, and their application should be evaluated in animal models. This review intends to discuss advanced TE methods for treating MMC and recent successes in increasing the efficacy of the treatment.
Graphical Abstract



Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- NTD:
-
Neural tube defect
- MMC:
-
Myelomeningocele
- MOMS:
-
Management of Myelomeningocele Study
- VP:
-
Ventriculoperitoneal
- TE:
-
Tissue engineering
- TRASCET:
-
Trans-amniotic stem cell therapy
- CSF:
-
Cerebrospinal fluid
- ADM:
-
Acellular dermal matrix
- bFGF:
-
Basic fibroblast growth factor
- AM:
-
Amniotic membrane
- PMSC:
-
Placenta-derived mesenchymal stem cell
- HUC:
-
Human umbilical cord
- BCF:
-
Biocellulose film
- PLA:
-
Polylactic acid
- PCL:
-
Polycaprolactone
- MSC:
-
Mesenchymal stem cell
- AFMSC:
-
Amniotic fluid-derived mesenchymal stem cell
- MHC:
-
Major Histocompatibility Complex
- BDNF:
-
Brain-derived neurotropic factor
- BMSC:
-
Bone marrow-derived mesenchymal stem cell
- ECM:
-
Extracellular matrix
- SLR:
-
Sheep Locomotor Rating
- NGF:
-
Nerve growth factor
- PDGF:
-
Platelet-derived growth factor
- EGF:
-
Epithelial growth factor
- TGF- α:
-
Transforming growth factor-α
- FGF:
-
Fibroblast growth factor
- iPSC:
-
Induced pluripotent stem cell
- VEGF:
-
Vascular endothelial growth factor
- MRI:
-
Magnetic resonance imaging
- PROM:
-
Prelabor rupture of membrane
- RTG:
-
Reverse thermal gel
References
Moldenhauer, J. S., & Flake, A. W. (2019). Open fetal surgery for neural tube defects. Best Practice & Research. Clinical Obstetrics & Gynaecology, 58, 121–132. https://doi.org/10.1016/j.bpobgyn.2019.03.004.
Sacco, A., Ushakov, F., Thompson, D., Peebles, D., Pandya, P., De Coppi, P., Wimalasundera, R., Attilakos, G., David, A. L., & Deprest, J. (2019). Fetal surgery for open spina bifida. The Obstetrician & Gynaecologist : the journal for continuing professional development from the Royal College of Obstetricians & Gynaecologists, 21(4), 271–282. https://doi.org/10.1111/tog.12603.
Khoshnood, B., Loane, M., Walle, H. d., Arriola, L., Addor, M.-C., Barisic, I., Beres, J., Bianchi, F., Dias, C., Draper, E., Garne, E., Gatt, M., Haeusler, M., Klungsoyr, K., Latos-Bielenska, A., Lynch, C., McDonnell, B., Nelen, V., Neville, A. J., O’Mahony, M. T., Queisser-Luft, A., Rankin, J., Rissmann, A., Ritvanen, A., Rounding, C., Sipek, A., Tucker, D., Verellen-Dumoulin, C., Wellesley, D., & Dolk, H. (2015). Long term trends in prevalence of neural tube defects in Europe: Population based study. BMJ, 351, h5949. https://doi.org/10.1136/bmj.h5949.
Kabagambe, S., Keller, B., Becker, J., Goodman, L., Pivetti, C., Lankford, L., Chung, K., Lee, C., Chen, Y. J., Kumar, P., Vanover, M., Wang, A., & Farmer, D. (2017). Placental mesenchymal stromal cells seeded on clinical grade extracellular matrix improve ambulation in ovine myelomeningocele. Journal of Pediatric Surgery, 53, 178–182. https://doi.org/10.1016/j.jpedsurg.2017.10.032.
Abe, Y., Ochiai, D., Masuda, H., Sato, Y., Otani, T., Fukutake, M., Ikenoue, S., Miyakoshi, K., Okano, H., & Tanaka, M. (2019). In utero amniotic fluid stem cell therapy protects against Myelomeningocele via spinal cord coverage and hepatocyte growth factor secretion. Stem Cells Translational Medicine, 8(11), 1170–1179. https://doi.org/10.1002/sctm.19-0002.
Guilbaud, L., Roux, N., Friszer, S., Garabedian, C., Dhombres, F., Bessières, B., Fallet-Bianco, C., Di Rocco, F., Zerah, M., & Jouannic, J. M. (2017). Fetoscopic patch coverage of experimental myelomenigocele using a two-port access in fetal sheep. Child's Nervous System : ChNS : official journal of the International Society for Pediatric Neurosurgery, 33(7), 1177–1184. https://doi.org/10.1007/s00381-017-3461-7.
Meuli, M., Meuli-Simmen, C., Flake, A. W., Zimmermann, R., Ochsenbein, N., Scheer, I., Mazzone, L., & Moehrlen, U. (2013). Premiere use of Integra™ artificial skin to close an extensive fetal skin defect during open in utero repair of myelomeningocele. Pediatric Surgery International, 29(12), 1321–1326. https://doi.org/10.1007/s00383-013-3412-7.
Joyeux, L., De Bie, F., Danzer, E., Van Mieghem, T., Flake, A. W., & Deprest, J. (2018). Safety and efficacy of fetal surgery techniques to close a spina bifida defect in the fetal lamb model: A systematic review. Prenatal Diagnosis, 38(4), 231–242. https://doi.org/10.1002/pd.5222.
Adzick, N. S., Thom, E. A., Spong, C. Y., Brock, J. W., Burrows, P. K., Johnson, M. P., Howell, L. J., Farrell, J. A., Dabrowiak, M. E., Sutton, L. N., Gupta, N., Tulipan, N. B., D'Alton, M. E., & Farmer, D. L. (2011). A randomized trial of prenatal versus postnatal repair of Myelomeningocele. New England Journal of Medicine, 364(11), 993–1004. https://doi.org/10.1056/NEJMoa1014379.
Watanabe, M., Kim, A. G., & Flake, A. W. (2015). Tissue engineering strategies for fetal myelomeningocele repair in animal models. Fetal Diagnosis and Therapy, 37(3), 197–205. https://doi.org/10.1159/000362931.
Vu, T., Mann, L. K., Fletcher, S. A., Jain, R., Garnett, J., Tsao, K., Austin, M. T., Moise Jr., K. J., Johnson, A., Shah, M. N., & Papanna, R. (2019). Suture techniques and patch materials using an in-vitro model for watertight closure of in-utero spina bifida repair. Journal of Pediatric Surgery, 55, 726–731. https://doi.org/10.1016/j.jpedsurg.2019.05.024.
Papanna, R., Moise Jr., K. J., Mann, L. K., Fletcher, S., Schniederjan, R., Bhattacharjee, M. B., Stewart, R. J., Kaur, S., Prabhu, S. P., & Tseng, S. C. (2016). Cryopreserved human umbilical cord patch for in-utero spina bifida repair. Ultrasound in Obstetrics & Gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology, 47(2), 168–176. https://doi.org/10.1002/uog.15790.
Dionigi, B., Brazzo 3rd, J. A., Ahmed, A., Feng, C., Wu, Y., Zurakowski, D., & Fauza, D. O. (2015). Trans-amniotic stem cell therapy (TRASCET) minimizes Chiari-II malformation in experimental spina bifida. Journal of Pediatric Surgery, 50(6), 1037–1041. https://doi.org/10.1016/j.jpedsurg.2015.03.034.
Hii, L. Y., Sung, C. A., & Shaw, S. W. (2020). Fetal surgery and stem cell therapy for meningomyelocele. Current Opinion in Obstetrics & Gynecology, 32(2), 147–151. https://doi.org/10.1097/gco.0000000000000614.
Watanabe, M., Jo, J., Radu, A., Kaneko, M., Tabata, Y., & Flake, A. W. (2010). A tissue engineering approach for prenatal closure of myelomeningocele with gelatin sponges incorporating basic fibroblast growth factor. Tissue Engineering. Part A, 16(5), 1645–1655. https://doi.org/10.1089/ten.TEA.2009.0532.
Tabata, Y. (2009). Biomaterial technology for tissue engineering applications. Journal of the Royal Society, Interface, 6 Suppl, 3(Suppl 3), S311–S324. https://doi.org/10.1098/rsif.2008.0448.focus.
Marwan, A. I., Williams, S. M., Bardill, J. R., Gralla, J., Abdul-Aziz, N. M., & Park, D. (2017). Reverse thermal gel for in utero coverage of Spina bifida defects: An innovative bioengineering alternative to open fetal repair. Macromolecular Bioscience, 17(6). https://doi.org/10.1002/mabi.201600473.
Tatu, R., Oria, M., Pulliam, S., Signey, L., Rao, M. B., Peiro, J. L., & Lin, C. Y. (2019). Using poly(l-lactic acid) and poly(ɛ-caprolactone) blends to fabricate self-expanding, watertight and biodegradable surgical patches for potential fetoscopic myelomeningocele repair. Journal of Biomedical Materials Research. Part B, Applied Biomaterials, 107(2), 295–305. https://doi.org/10.1002/jbm.b.34121.
Oria, M., Tatu, R. R., Lin, C. Y., & Peiro, J. L. (2019). In vivo evaluation of novel PLA/PCL polymeric patch in rats for potential Spina bifida coverage. The Journal of Surgical Research, 242, 62–69. https://doi.org/10.1016/j.jss.2019.04.035.
Hutmacher, D. W. (2000). Scaffolds in tissue engineering bone and cartilage. Biomaterials, 21(24), 2529–2543. https://doi.org/10.1016/S0142-9612(00)00121-6.
Mann, L. K., Won, J. H., Trenton, N. J., Garnett, J., Snowise, S., Fletcher, S. A., Tseng, S. C. G., Diehl, M. R., & Papanna, R. (2019). Cryopreserved human umbilical cord versus acellular dermal matrix patches for in utero fetal spina bifida repair in a pregnant rat model. Journal of Neurosurgery. Spine, 32(2), 321–331. https://doi.org/10.3171/2019.7.Spine19468.
Hosper, N. A., Eggink, A. J., Roelofs, L. A., Wijnen, R. M., van Luyn, M. J., Bank, R. A, Harmsen, M. C., Geutjes, P. J., Daamen, W. F., van Kuppevelt, T. H., Tiemessen, D. M., Oosterwijk, E., Crevels, J. J., Blokx, W. A., Lotgering, F. K., van den Berg, P. P., & Feitz, W. F. (2010). Intra-uterine tissue engineering of full-thickness skin defects in a fetal sheep model. Biomaterials, 31(14), 3910–3919. https://doi.org/10.1016/j.biomaterials.2010.01.129.
Saadai, P., Nout, Y. S., Encinas, J., Wang, A., Downing, T. L., Beattie, M. S., Bresnahan, J. C., Li, S., & Farmer, D. L. (2011). Prenatal repair of myelomeningocele with aligned nanofibrous scaffolds-a pilot study in sheep. Journal of Pediatric Surgery, 46(12), 2279–2283. https://doi.org/10.1016/j.jpedsurg.2011.09.014.
Fontecha, C. G., Peiro, J. L., Sevilla, J. J., Aguirre, M., Soldado, F., Fresno, L., Fonseca, C., Chacaltana, A., & Martinez, V. (2011). Fetoscopic coverage of experimental myelomeningocele in sheep using a patch with surgical sealant. European Journal of Obstetrics, Gynecology, and Reproductive Biology, 156(2), 171–176. https://doi.org/10.1016/j.ejogrb.2010.12.046.
Watanabe, M., Li, H., Roybal, J., Santore, M., Radu, A., Jo, J., Kaneko, M., Tabata, Y., & Flake, A. (2011). A tissue engineering approach for prenatal closure of myelomeningocele: Comparison of gelatin sponge and microsphere scaffolds and bioactive protein coatings. Tissue Engineering. Part A, 17(7–8), 1099–1110. https://doi.org/10.1089/ten.TEA.2010.0390.
Peiro, J. L., Fontecha, C. G., Ruano, R., Esteves, M., Fonseca, C., Marotta, M., Haeri, S., & Belfort, M. A. (2013). Single-access fetal endoscopy (SAFE) for myelomeningocele in sheep model I: Amniotic carbon dioxide gas approach. Surgical Endoscopy, 27(10), 3835–3840. https://doi.org/10.1007/s00464-013-2984-6.
Brown, E. G., Saadai, P., Pivetti, C. D., Beattie, M. S., Bresnahan, J. C., Wang, A., & Farmer, D. L. (2014). In utero repair of myelomeningocele with autologous amniotic membrane in the fetal lamb model. Journal of Pediatric Surgery, 49(1), 133–137discussion 137-138. https://doi.org/10.1016/j.jpedsurg.2013.09.043.
Watanabe, M., Li, H., Kim, A. G., Weilerstein, A., Radu, A., Davey, M., Loukogeorgakis, S., Sánchez, M. D., Sumita, K., Morimoto, N., Yamamoto, M., Tabata, Y., & Flake, A. W. (2016). Complete tissue coverage achieved by scaffold-based tissue engineering in the fetal sheep model of Myelomeningocele. Biomaterials, 76, 133–143. https://doi.org/10.1016/j.biomaterials.2015.10.051.
Papanna, R., Mann, L. K., Snowise, S., Morales, Y., Prabhu, S. P., Tseng, S. C., Grill, R., Fletcher, S., & Moise Jr., K. J. (2016). Neurological outcomes after human umbilical cord patch for in utero Spina bifida repair in a sheep model. AJP Reports, 6(3), e309–e317. https://doi.org/10.1055/s-0036-1592316.
Snowise, S., Mann, L., Morales, Y., Moise Jr., K. J., Johnson, A., Fletcher, S., Grill, R. J., Tseng, S. C. G., & Papanna, R. (2017). Cryopreserved human umbilical cord versus biocellulose film for prenatal spina bifida repair in a physiologic rat model. Prenatal Diagnosis, 37(5), 473–481. https://doi.org/10.1002/pd.5035.
Farrelly, J. S., Bianchi, A. H., Ricciardi, A. S., Buzzelli, G. L., Ahle, S. L., Freedman-Weiss, M. R., Luks, V. L., Saltzman, W. M., & Stitelman, D. H. (2019). Alginate microparticles loaded with basic fibroblast growth factor induce tissue coverage in a rat model of myelomeningocele. Journal of Pediatric Surgery, 54(1), 80–85. https://doi.org/10.1016/j.jpedsurg.2018.10.031.
Bardill, J., Williams, S. M., Shabeka, U., Niswander, L., Park, D., & Marwan, A. I. (2019). An injectable reverse thermal gel for minimally invasive coverage of mouse Myelomeningocele. The Journal of Surgical Research, 235, 227–236. https://doi.org/10.1016/j.jss.2018.09.078.
Bardill, J. R., Park, D., & Marwan, A. I. (2020). Improved coverage of mouse Myelomeningocele with a mussel inspired reverse thermal gel. The Journal of Surgical Research, 251, 262–274. https://doi.org/10.1016/j.jss.2020.01.022.
Mamede, A. C., Carvalho, M. J., Abrantes, A. M., Laranjo, M., Maia, C. J., & Botelho, M. F. (2012). Amniotic membrane: From structure and functions to clinical applications. Cell and Tissue Research, 349(2), 447–458. https://doi.org/10.1007/s00441-012-1424-6.
Kogan, S., Sood, A., & Granick, M. S. (2018). Amniotic membrane adjuncts and clinical applications in wound healing: A review of the literature. Wounds, 30(6), 168–173.
Cooke, M., Tan, E. K., Mandrycky, C., He, H., O'Connell, J., & Tseng, S. C. (2014). Comparison of cryopreserved amniotic membrane and umbilical cord tissue with dehydrated amniotic membrane/chorion tissue. Journal of Wound Care, 23(10), 465–474, 476. https://doi.org/10.12968/jowc.2014.23.10.465.
Pedreira, D. A., Quintero, R. A., Acácio, G. L., Caldini, E. T., & Saldiva, P. H. (2011). Neoskin development in the fetus with the use of a three-layer graft: An animal model for in utero closure of large skin defects. The Journal of Maternal-Fetal & Neonatal Medicine, 24(10), 1243–1248. https://doi.org/10.3109/14767058.2011.564486.
Pedreira, D. A., Zanon, N., Nishikuni, K., Moreira de Sá, R. A., Acacio, G. L., Chmait, R. H., Kontopoulos, E. V., & Quintero, R. A. (2016). Endoscopic surgery for the antenatal treatment of myelomeningocele: The CECAM trial. American Journal of Obstetrics and Gynecology, 214(1), 111.e111. https://doi.org/10.1016/j.ajog.2015.09.065.
Mazzone, L., Pontiggia, L., Reichmann, E., Ochsenbein-Kölble, N., Moehrlen, U., & Meuli, M. (2014). Experimental tissue engineering of fetal skin. Pediatric Surgery International, 30(12), 1241–1247. https://doi.org/10.1007/s00383-014-3614-7.
Chen, Y. J., Chung, K., Pivetti, C., Lankford, L., Kabagambe, S. K., Vanover, M., Becker, J., Lee, C., Tsang, J., Wang, A., & Farmer, D. L. (2017). Fetal surgical repair with placenta-derived mesenchymal stromal cell engineered patch in a rodent model of myelomeningocele. Journal of Pediatric Surgery, 53, 183–188. https://doi.org/10.1016/j.jpedsurg.2017.10.040.
Vanover, M., Pivetti, C., Lankford, L., Kumar, P., Galganski, L., Kabagambe, S., Keller, B., Becker, J., Chen, Y. J., Chung, K., Lee, C., Paxton, Z., Deal, B., Goodman, L., Anderson, J., Jensen, G., Wang, A., & Farmer, D. (2019). High density placental mesenchymal stromal cells provide neuronal preservation and improve motor function following in utero treatment of ovine myelomeningocele. Journal of Pediatric Surgery, 54(1), 75–79. https://doi.org/10.1016/j.jpedsurg.2018.10.032.
Lee, D. H., Phi, J. H., Kim, S. K., Cho, B. K., Kim, S. U., & Wang, K. C. (2010). Enhanced reclosure of surgically induced spinal open neural tube defects in chick embryos by injecting human bone marrow stem cells into the amniotic cavity. Neurosurgery, 67(1), 129–135discussion 135. https://doi.org/10.1227/01.Neu.0000371048.76494.0f.
Li, H., Gao, F., Ma, L., Jiang, J., Miao, J., Jiang, M., Fan, Y., Wang, L., Wu, D., Liu, B., Wang, W., Lui, V. C., & Yuan, Z. (2012). Therapeutic potential of in utero mesenchymal stem cell (MSCs) transplantation in rat foetuses with spina bifida aperta. Journal of Cellular and Molecular Medicine, 16(7), 1606–1617. https://doi.org/10.1111/j.1582-4934.2011.01470.x.
Turner, C. G., Pennington, E. C., Gray, F. L., Ahmed, A., Teng, Y. D., & Fauza, D. O. (2013). Intra-amniotic delivery of amniotic-derived neural stem cells in a syngeneic model of Spina bifida. Fetal Diagnosis and Therapy, 34(1), 38–43. https://doi.org/10.1159/000350267.
Saadai, P., Wang, A., Nout, Y. S., Downing, T. L., Lofberg, K., Beattie, M. S., Bresnahan, J. C., Li, S., & Farmer, D. L. (2013). Human induced pluripotent stem cell-derived neural crest stem cells integrate into the injured spinal cord in the fetal lamb model of myelomeningocele. Journal of Pediatric Surgery, 48(1), 158–163. https://doi.org/10.1016/j.jpedsurg.2012.10.034.
Ma, W., Wei, X., Gu, H., Li, H., Guan, K., Liu, D., Chen, L., Cao, S., An, D., Zhang, H., Huang, T., Miao, J., Zhao, G., Wu, D., Liu, B., Wang, W., & Yuan, Z. (2015). Sensory neuron differentiation potential of in utero mesenchymal stem cell transplantation in rat fetuses with spina bifida aperta. Birth defects research Part A, Clinical and molecular teratology, 103(9), 772–779. https://doi.org/10.1002/bdra.23401.
Wang, A., Brown, E. G., Lankford, L., Keller, B. A., Pivetti, C. D., Sitkin, N. A., Beattie, M. S., Bresnahan, J. C., & Farmer, D. L. (2015). Placental mesenchymal stromal cells rescue ambulation in ovine myelomeningocele. Stem Cells Translational Medicine, 4(6), 659–669. https://doi.org/10.5966/sctm.2014-0296.
Li, X., Yuan, Z., Wei, X., Li, H., Zhao, G., Miao, J., Wu, D., Liu, B., Cao, S., An, D., Ma, W., Zhang, H., Wang, W., Wang, Q., & Gu, H. (2016). Application potential of bone marrow mesenchymal stem cell (BMSCs) based tissue-engineering for spinal cord defect repair in rat fetuses with spina bifida aperta. Journal of Materials Science. Materials in Medicine, 27(4), 77. https://doi.org/10.1007/s10856-016-5684-7.
Feng, C., Graham, C. D., Connors, J. P., Brazzo 3rd, J., Zurakowski, D., & Fauza, D. O. (2016). A comparison between placental and amniotic mesenchymal stem cells for transamniotic stem cell therapy (TRASCET) in experimental spina bifida. Journal of Pediatric Surgery, 51(6), 1010–1013. https://doi.org/10.1016/j.jpedsurg.2016.02.071.
Brown, E. G., Keller, B. A., Lankford, L., Pivetti, C. D., Hirose, S., Farmer, D. L., & Wang, A. (2016). Age does matter: A pilot comparison of placenta-derived stromal cells for in utero repair of Myelomeningocele using a lamb model. Fetal Diagnosis and Therapy, 39(3), 179–185. https://doi.org/10.1159/000433427.
Kajiwara, K., Tanemoto, T., Wada, S., Karibe, J., Ihara, N., Ikemoto, Y., Kawasaki, T., Oishi, Y., Samura, O., Okamura, K., Takada, S., Akutsu, H., Sago, H., Okamoto, A., & Umezawa, A. (2017). Fetal therapy model of Myelomeningocele with three-dimensional skin using amniotic fluid cell-derived induced pluripotent stem cells. Stem Cell Reports, 8(6), 1701–1713. https://doi.org/10.1016/j.stemcr.2017.05.013.
Shieh, H. F., Tracy, S. A., Hong, C. R., Chalphin, A. V., Ahmed, A., Rohrer, L., Zurakowski, D., & Fauza, D. O. (2019). Transamniotic stem cell therapy (TRASCET) in a rabbit model of spina bifida. Journal of Pediatric Surgery, 54(2), 293–296. https://doi.org/10.1016/j.jpedsurg.2018.10.086.
Vanover, M., Pivetti, C., Galganski, L., Kumar, P., Lankford, L., Rowland, D., Paxton, Z., Deal, B., Wang, A., & Farmer, D. (2019). Spinal angulation: A limitation of the fetal lamb model of Myelomeningocele. Fetal Diagnosis and Therapy, 46(6), 376–384. https://doi.org/10.1159/000496201.
Galganski, L. A., Kumar, P., Vanover, M. A., Pivetti, C. D., Anderson, J. E., Lankford, L., Paxton, Z. J., Chung, K., Lee, C., Hegazi, M. S., Yamashiro, K. J., Wang, A., & Farmer, D. L. (2019). In utero treatment of Myelomeningocele with placental Mesenchymal stromal cells - selection of an optimal cell line in preparation for clinical trials. Journal of Pediatric Surgery. https://doi.org/10.1016/j.jpedsurg.2019.09.029.
Mazzone, L., Moehrlen, U., Ochsenbein-Kölble, N., Pontiggia, L., Biedermann, T., Reichmann, E., & Meuli, M. (2020). Bioengineering and in utero transplantation of fetal skin in the sheep model: A crucial step towards clinical application in human fetal spina bifida repair. Journal of Tissue Engineering and Regenerative Medicine, 14(1), 58–65. https://doi.org/10.1002/term.2963.
Dionigi, B., Ahmed, A., Brazzo 3rd, J., Connors, J. P., Zurakowski, D., & Fauza, D. O. (2015). Partial or complete coverage of experimental spina bifida by simple intra-amniotic injection of concentrated amniotic mesenchymal stem cells. Journal of Pediatric Surgery, 50(1), 69–73. https://doi.org/10.1016/j.jpedsurg.2014.10.004.
Brown, C., McKee, C., Bakshi, S., Walker, K., Hakman, E., Halassy, S., Svinarich, D., Dodds, R., Govind, C. K., & Chaudhry, G. R. (2019). Mesenchymal stem cells: Cell therapy and regeneration potential. Journal of Tissue Engineering and Regenerative Medicine, 13(9), 1738–1755. https://doi.org/10.1002/term.2914.
Joerger-Messerli, M. S., Marx, C., Oppliger, B., Mueller, M., Surbek, D. V., & Schoeberlein, A. (2016). Mesenchymal stem cells from Wharton's jelly and amniotic fluid. Best Practice & Research. Clinical Obstetrics & Gynaecology, 31, 30–44. https://doi.org/10.1016/j.bpobgyn.2015.07.006.
Moschidou, D., Mukherjee, S., Blundell, M. P., Jones, G. N., Atala, A. J., Thrasher, A. J., Fisk, N. M., De Coppi, P., & Guillot, P. V. (2013). Human mid-trimester amniotic fluid stem cells cultured under embryonic stem cell conditions with valproic acid acquire pluripotent characteristics. Stem Cells and Development, 22(3), 444–458. https://doi.org/10.1089/scd.2012.0267.
Ceccarelli, G., Pozzo, E., Scorletti, F., Benedetti, L., Cusella, G., Ronzoni, F. L., Sahakyan, V., Zambaiti, E., Mimmi, M. C., Calcaterra, V., Deprest, J., Sampaolesi, M., & Pelizzo, G. (2015). Molecular signature of amniotic fluid derived stem cells in the fetal sheep model of myelomeningocele. Journal of Pediatric Surgery, 50(9), 1521–1527. https://doi.org/10.1016/j.jpedsurg.2015.04.014.
Hosper, N. A., Bank, R. A, & van den Berg, P. P. (2014). Human amniotic fluid-derived mesenchymal cells from fetuses with a neural tube defect do not deposit collagen type i protein after TGF-β1 stimulation in vitro. Stem Cells and Development, 23(5), 555–562. https://doi.org/10.1089/scd.2013.0334.
Basler, M., Pontiggia, L., Biedermann, T., Reichmann, E., Meuli, M., & Mazzone, L. (2020). Bioengineering of fetal skin: Differentiation of amniotic fluid stem cells into keratinocytes. Fetal Diagnosis and Therapy, 47(3), 198–204. https://doi.org/10.1159/000502181.
Lee, J. M., Jung, J., Lee, H.-J., Jeong, S. J., Cho, K. J., Hwang, S.-G., & Kim, G. J. (2012). Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. International Immunopharmacology, 13(2), 219–224. https://doi.org/10.1016/j.intimp.2012.03.024.
Somuncu, Ö. S., Coşkun, Y., Ballica, B., Temiz, A. F., & Somuncu, D. (2019). In vitro artificial skin engineering by decellularized placental scaffold for secondary skin problems of meningomyelocele. Journal of Clinical Neuroscience : official journal of the Neurosurgical Society of Australasia, 59, 291–297. https://doi.org/10.1016/j.jocn.2018.10.044.
Cao, S., Wei, X., Li, H., Miao, J., Zhao, G., Wu, D., Liu, B., Zhang, Y., Gu, H., Wang, L., Fan, Y., An, D., & Yuan, Z. (2016). Comparative study on the differentiation of Mesenchymal stem cells between fetal and postnatal rat spinal cord niche. Cell Transplantation, 25(6), 1115–1130. https://doi.org/10.3727/096368915x689910.
O'Brien, F. J. (2011). Biomaterials & scaffolds for tissue engineering. Materials Today, 14(3), 88–95. https://doi.org/10.1016/S1369-7021(11)70058-X.
Liang, X., Ding, Y., Zhang, Y., Tse, H. F., & Lian, Q. (2014). Paracrine mechanisms of mesenchymal stem cell-based therapy: Current status and perspectives. Cell Transplantation, 23(9), 1045–1059. https://doi.org/10.3727/096368913x667709.
Meuli, M., Meuli-Simmen, C., Yingling, C. D., Hutchins, G. M., Hoffman, K. M., Harrison, M. R., & Adzick, N. S. (1995). Creation of myelomeningocele in utero: A model of functional damage from spinal cord exposure in fetal sheep. Journal of Pediatric Surgery, 30(7), 1028–1032discussion 1032-1023. https://doi.org/10.1016/0022-3468(95)90335-6.
Guilbaud, L., Garabedian, C., Di Rocco, F., Fallet-Bianco, C., Friszer, S., Zerah, M., & Jouannic, J. M. (2014). Limits of the surgically induced model of myelomeningocele in the fetal sheep. Child's Nervous System : ChNS : official journal of the International Society for Pediatric Neurosurgery, 30(8), 1425–1429. https://doi.org/10.1007/s00381-014-2426-3.
Lopez de Torre, B., Tovar, J. A., Aldazabal, P., Uriarte, S., Rey, A., Ruiz, I., & San Vicente, M. (1990). Spina bifida: A chick embryo experimental model. Zeitschrift für Kinderchirurgie, 45(Suppl 1), 20–22. https://doi.org/10.1055/s-2008-1042628.
Housley, H. T., Graf, J. L., Lipshultz, G. S., Calvano, C. J., Harrison, M. R., Farmer, D. L., & Jennings, R. W. (2000). Creation of myelomeningocele in the fetal rabbit. Fetal Diagnosis and Therapy, 15(5), 275–279. https://doi.org/10.1159/000021021.
Danzer, E., Schwarz, U., Wehrli, S., Radu, A., Adzick, N. S., & Flake, A. W. (2005). Retinoic acid induced myelomeningocele in fetal rats: Characterization by histopathological analysis and magnetic resonance imaging. Experimental Neurology, 194(2), 467–475. https://doi.org/10.1016/j.expneurol.2005.03.011.
Yamashiro, K. J., Galganski, L. A., & Hirose, S. (2019). Fetal myelomeningocele repair. Seminars in Pediatric Surgery, 28(4), 150823. https://doi.org/10.1053/j.sempedsurg.2019.07.006.
Li, H., Miao, J., Zhao, G., Wu, D., Liu, B., Wei, X., Cao, S., Gu, H., Zhang, Y., Wang, L., Fan, Y., & Yuan, Z. (2014). Different expression patterns of growth factors in rat fetuses with spina bifida aperta after in utero mesenchymal stromal cell transplantation. Cytotherapy, 16(3), 319–330. https://doi.org/10.1016/j.jcyt.2013.10.005.
Sirico, A., Raffone, A., Lanzone, A., Saccone, G., Travaglino, A., Sarno, L., Rizzo, G., Zullo, F., & Maruotti, G. M. (2020). First trimester detection of fetal open spina bifida using BS/BSOB ratio. Archives of Gynecology and Obstetrics, 301(2), 333–340. https://doi.org/10.1007/s00404-019-05422-3.
Munoz, J. L., Bishop, E., Reider, M., Radeva, M., & Singh, K. (2019). Antenatal ultrasound compared to MRI evaluation of fetal myelomeningocele: A prenatal and postnatal evaluation. Journal of Perinatal Medicine, 47(7), 771–774. https://doi.org/10.1515/jpm-2019-0177.
Lapa, D. A. (2019). Endoscopic fetal surgery for neural tube defects. Best Practice & Research. Clinical Obstetrics & Gynaecology, 58, 133–141. https://doi.org/10.1016/j.bpobgyn.2019.05.001.
Adzick, N. S. (2012). Fetal surgery for myelomeningocele: Trials and tribulations. Isabella Forshall lecture. Journal of Pediatric Surgery, 47(2), 273–281. https://doi.org/10.1016/j.jpedsurg.2011.11.021.
Meuli, M., & Moehrlen, U. (2014). Fetal surgery for myelomeningocele is effective: A critical look at the whys. Pediatric Surgery International, 30(7), 689–697. https://doi.org/10.1007/s00383-014-3524-8.
Kabagambe, S. K., Jensen, G. W., Chen, Y. J., Vanover, M. A., & Farmer, D. L. (2018). Fetal surgery for Myelomeningocele: A systematic review and meta-analysis of outcomes in Fetoscopic versus open repair. Fetal Diagnosis and Therapy, 43(3), 161–174. https://doi.org/10.1159/000479505.
Freedman-Weiss, M. R., & Stitelman, D. H. (2020). Minimally invasive fetal therapy for Myelomeningocele. Current Stem Cell Reports, 6(1), 1–5. https://doi.org/10.1007/s40778-020-00167-1.
Heuer, G. G., Moldenhauer, J. S., & Scott Adzick, N. (2017). Prenatal surgery for myelomeningocele: Review of the literature and future directions. Child's Nervous System : ChNS : official journal of the International Society for Pediatric Neurosurgery, 33(7), 1149–1155. https://doi.org/10.1007/s00381-017-3440-z.
Peranteau, W. H., & Adzick, N. S. (2016). Prenatal surgery for myelomeningocele. Current Opinion in Obstetrics & Gynecology, 28(2), 111–118. https://doi.org/10.1097/gco.0000000000000253.
Kahr, M. K., Winder, F. M., Vonzun, L., Mazzone, L., Moehrlen, U., Meuli, M., Hüsler, M., Krähenmann, F., Zimmermann, R., & Ochsenbein-Kölble, N. (2019). Open intrauterine fetal Myelomeningocele repair: Changes in the surgical procedure and perinatal complications during the first 8 years of experience at a single center. Fetal Diagnosis and Therapy, 1-6, 485–490. https://doi.org/10.1159/000503388.
Bruner, J. P., Richards, W. O., Tulipan, N. B., & Arney, T. L. (1999). Endoscopic coverage of fetal myelomeningocele in utero. American Journal of Obstetrics and Gynecology, 180(1 Pt 1), 153–158. https://doi.org/10.1016/s0002-9378(99)70167-5.
Farmer, D. L., von Koch, C. S., Peacock, W. J., Danielpour, M., Gupta, N., Lee, H., & Harrison, M. R. (2003). In utero repair of myelomeningocele: Experimental pathophysiology, initial clinical experience, and outcomes. Archives of Surgery, 138(8), 872–878. https://doi.org/10.1001/archsurg.138.8.872.
Kohl, T., Tchatcheva, K., Merz, W., Wartenberg, H. C., Heep, A., Müller, A., Franz, A., Stressig, R., Willinek, W., & Gembruch, U. (2009). Percutaneous fetoscopic patch closure of human spina bifida aperta: Advances in fetal surgical techniques may obviate the need for early postnatal neurosurgical intervention. Surgical Endoscopy, 23(4), 890–895. https://doi.org/10.1007/s00464-008-0153-0.
Carrabba, G., Macchini, F., Fabietti, I., Schisano, L., Meccariello, G., Campanella, R., Bertani, G., Locatelli, M., Boito, S., Porro, G. A., Gabetta, L., Picciolini, O., Cinnante, C., Triulzi, F., Ciralli, F., Mosca, F., Lapa, D. A., Leva, E., Rampini, P., & Persico, N. (2019). Minimally invasive fetal surgery for myelomeningocele: Preliminary report from a single center. Neurosurgical Focus, 47(4), E12. https://doi.org/10.3171/2019.8.Focus19438.
Lapa Pedreira, D. A., Acacio, G. L., Gonçalves, R. T., Sá, R. A. M., Brandt, R. A., Chmait, R. H., Kontopoulos, E. V., & Quintero, R. A. (2018). Percutaneous fetoscopic closure of large open spina bifida using a bilaminar skin substitute. Ultrasound in Obstetrics & Gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology, 52(4), 458–466. https://doi.org/10.1002/uog.19001.
Lazow, S. P., & Fauza, D. O. (2020). Transamniotic stem cell therapy. Advances in Experimental Medicine and Biology, 1237, 61–74. https://doi.org/10.1007/5584_2019_416.
Acknowledgments
Graphical abstract and both figures are created with BioRender.com
Author information
Authors and Affiliations
Contributions
ASK wrote the manuscript.
MMZ revised the manuscript.
A-MK and MS revised the final manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent to Publish
Not applicable.
Competing Interests
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
This article belongs to the Topical Collection: Special issue on Neurogenesis and Neurodegeneration: Basic Research and Clinic Applications
Guest Editor: Henning Ulrich
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 89 kb).
Rights and permissions
About this article
Cite this article
Soltani Khaboushan, A., Shakibaei, M., Kajbafzadeh, AM. et al. Prenatal Neural Tube Anomalies: A Decade of Intrauterine Stem Cell Transplantation Using Advanced Tissue Engineering Methods. Stem Cell Rev and Rep 18, 752–767 (2022). https://doi.org/10.1007/s12015-021-10150-w
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-021-10150-w