Abstract
Brown and beige adipocytes have been widely known for their potential to dissipate excessive energy into heat form, resulting in an alleviation of obesity and other overweight-related conditions. This review highlights the origins, characteristics, and functions of the various kinds of adipocytes, as well as their anatomic distribution inside the human body. This review mainly focuses on various essential transcriptional factors such as PRDM16, FGF21, PPARα, PPARγ and PGC-1α, which exert their effects on the development and activation of thermogenic adipocytes via important pathways such as JAK-STAT, cAMP-PKA and PI3K-AKT signaling pathways. Additionally, this review will underline promising strategies to generate an unexhausted source of thermogenic adipocytes differentiated from human stem cells. These exogenous thermogenic adipocytes offer therapeutic potential for improvement of metabolic disorders via application as single cell or whole tissue transplantation.

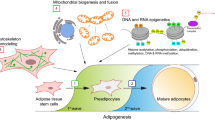
Caption is required. Please provide.


Similar content being viewed by others
Abbreviations
- ADSC:
-
adipose derived stem cell
- ATP:
-
adenosine triphosphate
- BA:
-
brown adipocytes
- BAT:
-
brown adipose tissue
- BMI:
-
body mass index
- BM-MSC:
-
bone marrow derived mesenchymal stem cell
- hESC:
-
human embryonic stem cell
- hiPSC:
-
human induced pluripotent stem cell
- MPC:
-
mesenchymal progenitor cell
- MSC:
-
mesenchymal stem cell
- UCB-MSC:
-
umbilical cord blood-derived mesenchymal stem cell
- WA:
-
white adipocytes
- WAT:
-
white adipose tissue
References
Hany, T. F., Gharehpapagh, E., Kamel, E. M., Buck, A., Himms-Hagen, J., & von Schulthess, G. K. (2002). Brown adipose tissue: a factor to consider in symmetrical tracer uptake in the neck and upper chest region. European Journal of Nuclear Medicine and Molecular Imaging, 29(10), 1393–1398.
Cypess, A. M., Lehman, S., Williams, G., Tal, I., Rodman, D., Goldfine, A. B., et al. (2009). Identification and importance of brown adipose tissue in adult humans. The New England Journal of Medicine, 360(15), 1509–1517.
Leitner, B. P., Huang, S., Brychta, R. J., Duckworth, C. J., Baskin, A. S., McGehee, S., et al. (2017). Mapping of human brown adipose tissue in lean and obese young men. Proceedings of the National Academy of Sciences of the United States of America, 114(32), 8649–8654.
Cypess, A. M., White, A. P., Vernochet, C., Schulz, T. J., Xue, R., Sass, C. A., et al. (2013). Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nature Medicine, 19(5), 635–639.
Soler-Vazquez, M. C., Mera, P., Zagmutt, S., Serra, D., & Herrero, L. (2018). New approaches targeting brown adipose tissue transplantation as a therapy in obesity. Biochemical Pharmacology, 155, 346–355.
Singh, A. M., & Dalton, S. (2018). What Can ‘Brown-ing’ Do For You? Trends in endocrinology and metabolism. TEM, 29(5), 349–359.
Yoneshiro, T., Aita, S., Matsushita, M., Kayahara, T., Kameya, T., Kawai, Y., et al. (2013). Recruited brown adipose tissue as an antiobesity agent in humans. The Journal of Clinical Investigation, 123(8), 3404–3408.
Chouchani, E. T., Kazak, L., & Spiegelman, B. M. (2019). New advances in adaptive thermogenesis: UCP1 and beyond. Cell Metabolism, 29(1), 27–37.
Fisher, F. M., Kleiner, S., Douris, N., Fox, E. C., Mepani, R. J., Verdeguer, F., et al. (2012). FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes & Development, 26(3), 271–281.
Berbee, J. F., Boon, M. R., Khedoe, P. P., Bartelt, A., Schlein, C., Worthmann, A., et al. (2015). Brown fat activation reduces hypercholesterolaemia and protects from atherosclerosis development. Nature Communications, 6, 6356.
Carobbio, S., Guenantin, A. C., Samuelson, I., Bahri, M., & Vidal-Puig, A. (2019). Brown and beige fat: From molecules to physiology and pathophysiology. Biochimica et Biophysica Acta Molecular and Cell Biology of Lipids, 1864(1), 37–50.
Cuevas-Ramos, D., Mehta, R., & Aguilar-Salinas, C. A. (2019). Fibroblast growth factor 21 and browning of white adipose tissue. Frontiers in Physiology, 10, 37.
Ikeda, K., Kang, Q., Yoneshiro, T., Camporez, J. P., Maki, H., Homma, M., et al. (2017). UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nature Medicine, 23(12), 1454–1465.
Chondronikola, M., Volpi, E., Borsheim, E., Porter, C., Saraf, M. K., Annamalai, P., et al. (2016). Brown adipose tissue activation is linked to distinct systemic effects on lipid metabolism in humans. Cell Metabolism, 23(6), 1200–1206.
Jung, S. M., Sanchez-Gurmaches, J., & Guertin, D. A. (2018) Brown adipose tissue development and metabolism. Handbook of experimental pharmacology.
Sacks, H., & Symonds, M. E. (2013). Anatomical locations of human brown adipose tissue: functional relevance and implications in obesity and type 2 diabetes. Diabetes, 62(6), 1783–1790.
Chi, J., Wu, Z., Choi, C. H. J., Nguyen, L., Tegegne, S., Ackerman, S. E., et al. (2018). Three-dimensional adipose tissue imaging reveals regional variation in beige fat biogenesis and PRDM16-dependent sympathetic neurite density. Cell Metabolism, 27(1), 226–36.e3.
Cohen, P., Levy, J. D., Zhang, Y., Frontini, A., Kolodin, D. P., Svensson, K. J., et al. (2014). Ablation of PRDM16 and beige adipose causes metabolic dysfunction and a subcutaneous to visceral fat switch. Cell, 156(1–2), 304–316.
Carobbio, S., Rosen, B., & Vidal-Puig, A. (2013). Adipogenesis: new insights into brown adipose tissue differentiation. Journal of Molecular Endocrinology, 51(3), T75–T85.
Sanchez-Gurmaches, J., & Guertin, D. A. (2014). Adipocyte lineages: tracing back the origins of fat. Biochimica et Biophysica Acta, 1842(3), 340–351.
Bargut, T. C. L., Souza-Mello, V., Aguila, M. B., & Mandarim-de-Lacerda, C. A. (2017) Browning of white adipose tissue: lessons from experimental models. Hormone Molecular Biology and Clinical Investigation, 31(1).
Kiefer, F. W. (2016). Browning and thermogenic programing of adipose tissue. Best Practice & Research Clinical Endocrinology & Metabolism, 30(4), 479–485.
Cedikova, M., Kripnerova, M., Dvorakova, J., Pitule, P., Grundmanova, M., Babuska, V., et al. (2016). Mitochondria in white, brown, and beige adipocytes. Stem Cells International, 2016, 6067349.
Cheng, Y., Jiang, L., Keipert, S., Zhang, S., Hauser, A., Graf, E., et al. (2018). Prediction of Adipose browning capacity by systematic integration of transcriptional profiles. Cell Reports, 23(10), 3112–3125.
Park, A., Kim, W. K., & Bae, K. H. (2014). Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World Journal of Stem Cells, 6(1), 33–42.
Schulz, T. J., & Tseng, Y. H. (2013). Brown adipose tissue: development, metabolism and beyond. The Biochemical Journal, 453(2), 167–178.
Seale, P., Conroe, H. M., Estall, J., Kajimura, S., Frontini, A., Ishibashi, J., et al. (2011). Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. The Journal of Clinical Investigation, 121(1), 96–105.
Farmer, S. R. (2006). Transcriptional control of adipocyte formation. Cell Metabolism, 4(4), 263–273.
Harms, M. J., Ishibashi, J., Wang, W., Lim, H. W., Goyama, S., Sato, T., et al. (2014). Prdm16 is required for the maintenance of brown adipocyte identity and function in adult mice. Cell Metabolism, 19(4), 593–604.
Roh, H. C., Tsai, L. T. Y., Shao, M., Tenen, D., Shen, Y., Kumari, M., et al. (2018). Warming induces significant reprogramming of beige, but not brown, adipocyte cellular identity. Cell Metabolism, 27(5), 1121–37.e5.
Sanchez-Gurmaches, J., Tang, Y., Jespersen, N. Z., Wallace, M., Martinez Calejman, C., Gujja, S., et al. (2018). Brown fat AKT2 is a cold-induced kinase that stimulates ChREBP-mediated de novo lipogenesis to optimize fuel storage and thermogenesis. Cell Metabolism, 27(1), 195-209.e6.
Razzoli, M., Emmett, M. J., Lazar, M. A., & Bartolomucci, A. (2018). beta-Adrenergic receptors control brown adipose UCP-1 tone and cold response without affecting its circadian rhythmicity. FASEB Journal: official publication of the Federation of American Societies for Experimental Biology, 32(10), 5640–5646.
Seale, P., Bjork, B., Yang, W., Kajimura, S., Chin, S., Kuang, S., et al. (2008). PRDM16 controls a brown fat/skeletal muscle switch. Nature, 454(7207), 961–967.
Hondares, E., Rosell, M., Diaz-Delfin, J., Olmos, Y., Monsalve, M., Iglesias, R., et al. (2011). Peroxisome proliferator-activated receptor alpha (PPARalpha) induces PPARgamma coactivator 1alpha (PGC-1alpha) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. The Journal of Biological Chemistry, 286(50), 43112–43122.
Floyd, Z. E., & Stephens, J. M. (2003). STAT5A promotes adipogenesis in nonprecursor cells and associates with the glucocorticoid receptor during adipocyte differentiation. Diabetes, 52(2), 308–314.
Richard, A. J., & Stephens, J. M. (2014). The role of JAK-STAT signaling in adipose tissue function. Biochimica et Biophysica acta, 1842(3), 431–439.
McGillicuddy, F. C., Chiquoine, E. H., Hinkle, C. C., Kim, R. J., Shah, R., Roche, H. M., et al. (2009). Interferon gamma attenuates insulin signaling, lipid storage, and differentiation in human adipocytes via activation of the JAK/STAT pathway. The Journal of Biological Chemistry, 284(46), 31936–31944.
O’Rourke, R. W., White, A. E., Metcalf, M. D., Winters, B. R., Diggs, B. S., Zhu, X., et al. (2012). Systemic inflammation and insulin sensitivity in obese IFN-gamma knockout mice. Metabolism: Clinical and Experimental, 61(8), 1152–1161.
Cernkovich, E. R., Deng, J., Bond, M. C., Combs, T. P., & Harp, J. B. (2008). Adipose-specific disruption of signal transducer and activator of transcription 3 increases body weight and adiposity. Endocrinology, 149(4), 1581–1590.
Derecka, M., Gornicka, A., Koralov, S. B., Szczepanek, K., Morgan, M., Raje, V., et al. (2012). Tyk2 and Stat3 regulate brown adipose tissue differentiation and obesity. Cell Metabolism, 16(6), 814–824.
Moisan, A., Lee, Y. K., Zhang, J. D., Hudak, C. S., Meyer, C. A., Prummer, M., et al. (2015). White-to-brown metabolic conversion of human adipocytes by JAK inhibition. Nature Cell Biology, 17(1), 57–67.
Keipert, S., Kutschke, M., Lamp, D., Brachthauser, L., Neff, F., Meyer, C. W., et al. (2015). Genetic disruption of uncoupling protein 1 in mice renders brown adipose tissue a significant source of FGF21 secretion. Molecular Metabolism, 4(7), 537–542.
Bernardo, B., Lu, M., Bandyopadhyay, G., Li, P., Zhou, Y., Huang, J., et al. (2015). FGF21 does not require interscapular brown adipose tissue and improves liver metabolic profile in animal models of obesity and insulin-resistance. Scientific Reports, 5, 11382.
Keipert, S., Kutschke, M., Ost, M., Schwarzmayr, T., van Schothorst, E. M., Lamp, D., et al. (2017). Long-term cold adaptation does not require FGF21 or UCP1. Cell Metabolism, 26(2), 437–46.e5.
Subash-Babu, P., & Alshatwi, A. A. (2018) Ononitol monohydrate enhances PRDM16 & UCP-1 expression, mitochondrial biogenesis and insulin sensitivity via STAT6 and LTB4R in maturing adipocytes. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 99, 375 – 83.
Piccinin, E., Morgano, A., Peres, C., Contursi, A., Bertrand-Michel, J., Arconzo, M., et al. (2019). PGC-1alpha induced browning promotes involution and inhibits lactation in mammary glands. Cellular: CMLS.
Tanaka-Yachi, R., Shirasaki, M., Otsu, R., Takahashi-Muto, C., Inoue, H., Aoki, Y., et al. (2018). delta-Tocopherol promotes thermogenic gene expression via PGC-1alpha upregulation in 3T3-L1 cells. Biochemical and Biophysical Research Communications, 506(1), 53–59.
Pettersson-Klein, A. T., Izadi, M., Ferreira, D. M. S., Cervenka, I., Correia, J. C., Martinez-Redondo, V., et al. (2018). Small molecule PGC-1alpha1 protein stabilizers induce adipocyte Ucp1 expression and uncoupled mitochondrial respiration. Molecular Metabolism, 9, 28–42.
Yan, Y., Yang, X., Zhao, T., Zou, Y., Li, R., & Xu, Y. (2017). Salicylates promote mitochondrial biogenesis by regulating the expression of PGC-1alpha in murine 3T3-L1 pre-adipocytes. Biochemical and Biophysical Research Communications, 491(2), 436–441.
Gettys, T. W., & Chang, J. S. (2019). An optimized immunoblotting protocol for accurate detection of endogenous PGC-1alpha isoforms in various rodent tissues. Methods in Molecular Biology (Clifton, NJ), 1966, 7–16.
Huang, P. I., Chen, Y. C., Chen, L. H., Juan, C. C., Ku, H. H., Wang, S. T., et al. (2011). PGC-1alpha mediates differentiation of mesenchymal stem cells to brown adipose cells. Journal of Atherosclerosis and Thrombosis, 18(11), 966–980.
Kleiner, S., Mepani, R. J., Laznik, D., Ye, L., Jurczak, M. J., Jornayvaz, F. R., et al. (2012). Development of insulin resistance in mice lacking PGC-1alpha in adipose tissues. Proceedings of the National Academy of Sciences of the United States of America, 109(24), 9635–9640.
Tang, Y., Wallace, M., Sanchez-Gurmaches, J., Hsiao, W. Y., Li, H., Lee, P. L., et al. (2016). Adipose tissue mTORC2 regulates ChREBP-driven de novo lipogenesis and hepatic glucose metabolism. Nature Communications, 7, 11365.
Seale, P., Kajimura, S., Yang, W., Chin, S., Rohas, L. M., Uldry, M., et al. (2007). Transcriptional control of brown fat determination by PRDM16. Cell Metabolism, 6(1), 38–54.
Jankovic, A., Golic, I., Markelic, M., Stancic, A., Otasevic, V., Buzadzic, B., et al. (2015). Two key temporally distinguishable molecular and cellular components of white adipose tissue browning during cold acclimation. The Journal of Physiology, 593(15), 3267–3280.
Schipper, H. S., Prakken, B., Kalkhoven, E., & Boes, M. (2012). Adipose tissue-resident immune cells: key players in immunometabolism. Trends in Endocrinology and Metabolism: TEM, 23(8), 407–415.
Nguyen, K. D., Qiu, Y., Cui, X., Goh, Y. P., Mwangi, J., David, T., et al. (2011). Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature, 480(7375), 104–108.
Pyrzak, B., Demkow, U., & Kucharska, A. M. (2015). Brown adipose tissue and browning agents: Irisin and FGF21 in the development of obesity in children and adolescents. Advances in Experimental Medicine and Biology, 866, 25–34.
Chartoumpekis, D. V., Habeos, I. G., Ziros, P. G., Psyrogiannis, A. I., Kyriazopoulou, V. E., & Papavassiliou, A. G. (2011). Brown adipose tissue responds to cold and adrenergic stimulation by induction of FGF21. Molecular Medicine, 17(7–8), 736–740.
Hondares, E., Iglesias, R., Giralt, A., Gonzalez, F. J., Giralt, M., Mampel, T., et al. (2011). Thermogenic activation induces FGF21 expression and release in brown adipose tissue. The Journal of Biological Chemistry, 286(15), 12983–12990.
Hanssen, M. J., Broeders, E., Samms, R. J., Vosselman, M. J., van der Lans, A. A., Cheng, C. C., et al. (2015). Serum FGF21 levels are associated with brown adipose tissue activity in humans. Scientific Reports, 5, 10275.
Schlein, C., Talukdar, S., Heine, M., Fischer, A. W., Krott, L. M., Nilsson, S. K., et al. (2016). FGF21 lowers plasma triglycerides by accelerating Lipoprotein Catabolism in white and brown adipose tissues. Cell Metabolism, 23(3), 441–453.
Park, Y. K., Wang, L., Giampietro, A., Lai, B., Lee, J. E., & Ge, K. (2017) Distinct roles of transcription factors KLF4, Krox20, and peroxisome proliferator-activated receptor gamma in adipogenesis. Molecular and Cellular Biology, 37(2).
Lasar, D., Rosenwald, M., Kiehlmann, E., Balaz, M., Tall, B., Opitz, L., et al. (2018). Peroxisome proliferator activated receptor gamma controls mature brown adipocyte inducibility through glycerol kinase. Cell Reports, 22(3), 760–773.
Fayyad, A. M., Khan, A. A., Abdallah, S. H., Alomran, S. S., Bajou, K., & Khattak, M. N. K. (2019) Rosiglitazone Enhances Browning Adipocytes in Association with MAPK and PI3-K Pathways During the Differentiation of Telomerase-Transformed Mesenchymal Stromal Cells into Adipocytes. International Journal of Molecular Sciences, 20(7).
Defour, M., Dijk, W., Ruppert, P., Nascimento, E. B. M., Schrauwen, P., & Kersten, S. (2018). The peroxisome proliferator-activated receptor alpha is dispensable for cold-induced adipose tissue browning in mice. Molecular Metabolism, 10, 39–54.
Xi, P., Xue, J., Wu, Z., Wang, H., Han, J., Liang, H., et al. (2019). Liver kinase B1 induces browning phenotype in 3T3-L1 adipocytes. Gene, 682, 33–41.
Krishnan, V., Baskaran, P., & Thyagarajan, B. (2019). Troglitazone activates TRPV1 and causes deacetylation of PPARgamma in 3T3-L1 cells. Biochimica et Biophysica Acta Molecular Basis of Disease, 1865(2), 445–453.
Kim, H. L., Park, J., Jung, Y., Ahn, K. S., Um, J. Y., & Platycodin, D. (2019). a novel activator of AMP-activated protein kinase, attenuates obesity in db/db mice via regulation of adipogenesis and thermogenesis. Phytomedicine: International Journal of Phytotherapy and Phytopharmacology, 52, 254–263.
Wei, S., Zhang, M., Zheng, Y., & Yan, P. (2018) ZBTB16 Overexpression enhances white adipogenesis and induces brown-like adipocyte formation of bovine white intramuscular preadipocytes. Cellular Physiology and Biochemistry: International Journal of Experimental Cellular Physiology, Biochemistry, and Pharmacology, 48(6), 2528–38.
Sim, C. K., Kim, S. Y., Brunmeir, R., Zhang, Q., Li, H., Dharmasegaran, D., et al. (2017). Regulation of white and brown adipocyte differentiation by RhoGAP DLC1. PLoS One, 12(3), e0174761.
Yamashita, Y., Mitani, T., Wang, L., & Ashida, H. (2018). Methylxanthine derivative-rich cacao extract suppresses differentiation of adipocytes through downregulation of PPARgamma and C/EBPs. Journal of Nutritional Science and Vitaminology, 64(2), 151–160.
Chang, J. S., & Ha, K. (2017). An unexpected role for the transcriptional coactivator isoform NT-PGC-1alpha in the regulation of mitochondrial respiration in brown adipocytes. The Journal of Biological Chemistry, 292(24), 9958–9966.
Shirvani, H., & Arabzadeh, E. (2018). Metabolic cross-talk between skeletal muscle and adipose tissue in high-intensity interval training vs. moderate-intensity continuous training by regulation of PGC-1alpha. Eating: EWD.
He, L., Tang, M., Xiao, T., Liu, H., Liu, W., Li, G., et al. (2018). Obesity-associated miR-199a/214 cluster inhibits adipose browning via PRDM16-PGC-1alpha transcriptional network. Diabetes, 67(12), 2585–2600.
Fang, P., He, B., Yu, M., Shi, M., Zhu, Y., Zhang, Z., et al. (2019). Treatment with celastrol protects against obesity through suppression of galanin-induced fat intake and activation of PGC-1alpha/GLUT4 axis-mediated glucose consumption. Biochimica et Biophysica Acta Molecular Basis of Disease, 1865(6), 1341–1350.
Singh, S. P., Huck, O., Abraham, N. G., & Amar, S. (2018) Kavain Reduces Porphyromonas gingivalis-Induced Adipocyte Inflammation: Role of PGC-1alpha Signaling. Journal of Immunology (Baltimore, Md: 1950), 201(5), 1491-9.
Choi, S. M., Tucker, D. F., Gross, D. N., Easton, R. M., DiPilato, L. M., Dean, A. S., et al. (2010). Insulin regulates adipocyte lipolysis via an Akt-independent signaling pathway. Molecular and Cellular Biology, 30(21), 5009–5020.
Shearin, A. L., Monks, B. R., Seale, P., & Birnbaum, M. J. (2016). Lack of AKT in adipocytes causes severe lipodystrophy. Molecular Metabolism, 5(7), 472–479.
Sanchez-Gurmaches, J., Martinez Calejman, C., Jung, S. M., Li, H., & Guertin, D. A. (2019). Brown fat organogenesis and maintenance requires AKT1 and AKT2. Molecular Metabolism, 23, 60–74.
Mundra, V., Gerling, I. C., & Mahato, R. I. (2013). Mesenchymal stem cell-based therapy. Molecular Pharmaceutics, 10(1), 77–89.
Wang, Y. L., Lin, S. P., Hsieh, P. C., & Hung, S. C. (2016). Concomitant beige adipocyte differentiation upon induction of mesenchymal stem cells into brown adipocytes. Biochemical and Biophysical Research Communications, 478(2), 689–695.
Yoneshiro, T., Shin, W., Machida, K., Fukano, K., Tsubota, A., Chen, Y., et al. (2019). Differentiation of bone marrow-derived cells toward thermogenic adipocytes in white adipose tissue induced by the beta3 adrenergic stimulation. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology, 33(4), 5196–5207.
Alessio, N., Squillaro, T., Monda, V., Peluso, G., Monda, M., Melone, M. A., et al. (2019). Circulating factors present in the sera of naturally skinny people may influence cell commitment and adipocyte differentiation of mesenchymal stromal cells. World Journal of Stem Cells., 11(3), 180–95.
Rashnonejad, A., Ercan, G., Gunduz, C., Akdemir, A., & Tiftikcioglu, Y. O. (2018). Comparative analysis of human UCB and adipose tissue derived mesenchymal stem cells for their differentiation potential into brown and white adipocytes. Molecular Biology Reports, 45(3), 233–244.
Wang, X., Chen, J., Rong, C., Pan, F., Zhao, X., & Hu, Y. (2018). GLP-1RA promotes brown adipogenesis of C3H10T1/2 mesenchymal stem cells via the PI3K-AKT-mTOR signaling pathway. Biochemical and Biophysical Research Communications, 506(4), 976–982.
Ahfeldt, T., Schinzel, R. T., Lee, Y. K., Hendrickson, D., Kaplan, A., Lum, D. H., et al. (2012). Programming human pluripotent stem cells into white and brown adipocytes. Nature Cell Biology, 14(2), 209–219.
Nishio, M., Yoneshiro, T., Nakahara, M., Suzuki, S., Saeki, K., Hasegawa, M., et al. (2012). Production of functional classical brown adipocytes from human pluripotent stem cells using specific hemopoietin cocktail without gene transfer. Cell Metabolism, 16(3), 394–406.
Mohsen-Kanson, T., Hafner, A. L., Wdziekonski, B., Takashima, Y., Villageois, P., Carriere, A., et al. (2014). Differentiation of human induced pluripotent stem cells into brown and white adipocytes: role of Pax3. Stem Cells (Dayton. Ohio), 32(6), 1459–1467.
Hafner, A. L., Contet, J., Ravaud, C., Yao, X., Villageois, P., Suknuntha, K., et al. (2016). Brown-like adipose progenitors derived from human induced pluripotent stem cells: Identification of critical pathways governing their adipogenic capacity. Scientific Reports, 6, 32490.
Guenantin, A. C., Briand, N., Capel, E., Dumont, F., Morichon, R., Provost, C., et al. (2017). Functional human beige adipocytes from induced pluripotent stem cells. Diabetes, 66(6), 1470–1478.
Nishikawa, S., Aoyama, H., Kamiya, M., Higuchi, J., Kato, A., Soga, M., et al. (2016). Artepillin C, a typical brazilian propolis-derived component, induces brown-like adipocyte formation in C3H10T1/2 cells, primary inguinal white adipose tissue-derived adipocytes, and mice. PLoS One, 11(9), e0162512.
Blumenfeld, N. R., Kang, H. J., Fenzl, A., Song, Z., Chung, J. J., Singh, R., et al. (2018). A direct tissue-grafting approach to increasing endogenous brown fat. Scientific Reports, 8(1), 7957.
Nishio, M., & Saeki, K. (2014). Differentiation of human pluripotent stem cells into highly functional classical brown adipocytes. Methods in Enzymology, 537, 177–197.
West, M. D., Chang, C. F., Larocca, D., Li, J., Jiang, J., Sim, P., et al. (2019). Clonal derivation of white and brown adipocyte progenitor cell lines from human pluripotent stem cells. Stem Cell Research & Therapy, 10(1), 7.
You, L., Zhou, Y., Cui, X., Wang, X., Sun, Y., Gao, Y., et al. (2018). GM13133 is a negative regulator in mouse white adipocytes differentiation and drives the characteristics of brown adipocytes. Journal of Cellular Physiology, 233(1), 313–324.
Xiong, Y., Yue, F., Jia, Z., Gao, Y., Jin, W., Hu, K., et al. (2018). A novel brown adipocyte-enriched long non-coding RNA that is required for brown adipocyte differentiation and sufficient to drive thermogenic gene program in white adipocytes. Biochimica et Biophysica Acta Molecular and Cell Biology of Lipids, 1863(4), 409–419.
Peng, X., Zhang, Q., Liao, C., Han, W., & Xu, F. (2018). Epigenomic Control of Thermogenic Adipocyte Differentiation and Function. International Journal of Molecular Sciences, 19(6).
Wu, X., Wang, Y., Wang, Y., Wang, X., Li, J., Chang, K., et al. (2018). GSK126 alleviates the obesity phenotype by promoting the differentiation of thermogenic beige adipocytes in diet-induced obese mice. Biochemical and Biophysical Research Communications, 501(1), 9–15.
Qi, G., Zhou, Y., Zhang, X., Yu, J., Li, X., Cao, X., et al. (2019). Cordycepin promotes browning of white adipose tissue through an AMP-activated protein kinase (AMPK)-dependent pathway. Acta Pharmaceutica Sinica B, 9(1), 135–143.
Ludwig, R. G., Rocha, A. L., & Mori, M. A. (2018). Circulating molecules that control brown/beige adipocyte differentiation and thermogenic capacity. Cell Biology International, 42(6), 701–710.
Lee, Y. H., Kim, S. N., Kwon, H. J., & Granneman, J. G. (2017). Metabolic heterogeneity of activated beige/brite adipocytes in inguinal adipose tissue. Scientific Reports, 7, 39794.
Dehghani, M., Kargarfard, M., Rabiee, F., Nasr-Esfahani, M. H., & Ghaedi, K. (2018). A comparative study on the effects of acute and chronic downhill running vs uphill running exercise on the RNA levels of the skeletal muscles PGC1-alpha, FNDC5 and the adipose UCP1 in BALB/c mice. Gene, 679, 369–376.
Berry, D. C., Jiang, Y., Arpke, R. W., Close, E. L., Uchida, A., Reading, D., et al. (2017). Cellular aging contributes to failure of cold-induced beige adipocyte formation in old mice and humans. Cell Metabolism, 25(1), 166–181.
Seale, P., & Lazar, M. A. (2009). Brown fat in humans: turning up the heat on obesity. Diabetes, 58(7), 1482–1484.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Van Nguyen, TT., Vu, V.V. & Pham, P.V. Transcriptional Factors of Thermogenic Adipocyte Development and Generation of Brown and Beige Adipocytes From Stem Cells. Stem Cell Rev and Rep 16, 876–892 (2020). https://doi.org/10.1007/s12015-020-10013-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-020-10013-w