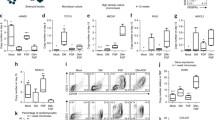
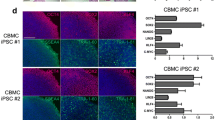
Abstract
The propensity of induced pluripotent stem (iPS) cells to differentiate into specific lineages may be influenced by a number of factors, including the selection of the somatic cell type used for reprogramming. Herein we report the generation of new iPS cells, which we derived from human articular chondrocytes and from cord blood mononucleocytes via lentiviral-mediated delivery of Oct4, Klf4, Sox2, and cMyc. Molecular, cytochemical, and cytogenic analyses confirmed the acquisition of hallmark features of pluripotency, as well as the retention of normal karyotypes following reprogramming of both the human articular chondrocytes (AC) and the cord blood (CB) cells. In vitro and in vivo functional analyses formally established the pluripotent differentiation capacity of all cell lines. Chondrogenic differentiation assays comparing iPS cells derived from AC, CB, and a well established dermal fibroblast cell line (HDFa-Yk26) identified enhanced proteoglycan-rich matrix formation and cartilage-associated gene expression from AC-derived iPS cells. These findings suggest that the tissue of origin may impact the fate potential of iPS cells for differentiating into specialized cell types, such as chondrocytes. Thus, we generated new cellular tools for the identification of inherent features driving high chondrogenic potential of reprogrammed cells.





Similar content being viewed by others
Abbreviations
- AC:
-
articular chondrocytes
- ALP:
-
alkaline phosphatase
- BMP-2:
-
bone morphogenetic protein-2
- CB:
-
cord blood
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- EB:
-
embryoid bodies
- ESC:
-
embryonic stem cell
- iPS cells:
-
induced pluripotent stem cells
- MEFs:
-
mouse embryonic fibroblasts
- SF:
-
skin fibroblasts
- SR:
-
serum replacement
References
Nakayama N. & Umeda K. (2011) From Pluripotent Stem Cells to Lineage-Specific Chondrocytes: Essential Signaling and Cellular Intermediates. Embryonic Stem Cells: The Hormonal Regulation of Pluripotency and Embryogenesis, ed Atwood C (InTech).
Robinton, D. A., & Daley, G. Q. (2012). The promise of induced pluripotent stem cells in research and therapy. Nature, 481, 295–305.
Wu, S. M., & Hochedlinger, K. (2011). Harnessing the potential of induced pluripotent stem cells for regenerative medicine. Nature Cell Biology, 13, 497–505.
Long, F., & Ornitz, D. M. (2013). Development of the endochondral skeleton. Cold Spring Harbor Perspectives in Biology, 5, a008334.
Lefebvre, V., & Smits, P. (2005). Transcriptional control of chondrocyte fate and differentiation. Birth Defects Research. Part C, Embryo Today, 75(3), 200–212.
Wuelling, M., & Vortkamp, A. (2010). Transcriptional networks controlling chondrocyte proliferation and differentiation during endochondral ossification. Pediatric Nephrology (Berlin, Germany), 25, 625–631.
Guzzo, R. M., Gibson, J., Xu, R. H., Lee, F. Y., & Drissi, H. (2013). Efficient differentiation of human iPSC-derived mesenchymal stem cells to chondroprogenitor cells. Journal of Cellular Biochemistry, 114, 480–490.
Toh, W. S., Lee, E. H., Richards, M., & Cao, T. (2010). In vitro derivation of chondrogenic cells from human embryonic stem cells. Methods in Molecular Biology, 584, 317–331.
Craft, A. M., Ahmed, N., Rockel, J. S., et al. (2013). Specification of chondrocytes and cartilage tissues from embryonic stem cells. Development (Cambridge, England), 140, 2597–2610.
Oldershaw, R. A., Baxter, M. A., Lowe, E. T., et al. (2010). Directed differentiation of human embryonic stem cells toward chondrocytes. Nature Biotechnology, 28, 1187–1194.
Diekman, B. O., Christoforou, N., Willard, V. P., et al. (2012). Cartilage tissue engineering using differentiated and purified induced pluripotent stem cells. Proceedings of the National Academy of Sciences of the United States of America, 109, 19172–19177.
Onyekwelu, I., Goldring, M. B., & Hidaka, C. (2009). Chondrogenesis, joint formation, and articular cartilage regeneration. Journal of Cellular Biochemistry, 107, 383–392.
Koyama, N., Miura, M., Nakao, K., et al. (2013). Human induced pluripotent stem cells differentiated into chondrogenic lineage via generation of mesenchymal progenitor cells. Stem Cells and Development, 22, 102–113.
Liu, Y., Goldberg, A. J., Dennis, J. E., Gronowicz, G. A., & Kuhn, L. T. (2012). One-step derivation of mesenchymal stem cell (MSC)-like cells from human pluripotent stem cells on a fibrillar collagen coating. PLoS One, 7(3), e33225.
Abyzov, A., Mariani, J., Palejev, D., et al. (2012). Somatic copy number mosaicism in human skin revealed by induced pluripotent stem cells. Nature, 492, 438–442.
Zhang, X. B. (2013). Cellular reprogramming of human peripheral blood cells. Genomics, Proteomics & Bioinformatics, 11, 264–274.
Meng, X., Neises, A., Su, R. J., et al. (2009). Efficient reprogramming of human cord blood CD34+ cells into induced pluripotent stem cells with OCT4 and SOX2 alone. Molecular Therapy, 20, 408–416.
Haase, A., Olmer, R., Schwanke, K., et al. (2009). Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell, 5, 434–441.
Bar-Nur, O., Russ, H. A., Efrat, S., & Benvenisty, N. (2011). Epigenetic memory and preferential lineage-specific differentiation in induced pluripotent stem cells derived from human pancreatic islet Beta cells. Cell Stem Cell, 9, 17–23.
Kim, K., Doi, A., Wen, B., et al. (2010). Epigenetic memory in induced pluripotent stem cells. Nature, 467, 285–290.
Kim, K., Zhao, R., Doi, A., et al. (2011). Donor cell type can influence the epigenome and differentiation potential of human induced pluripotent stem cells. Nature Biotechnology, 29, 1117–1119.
Polo, J. M., Liu, S., Figueroa, M. E., et al. (2010). Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nature Biotechnology, 28, 848–855.
Sullivan, G. J., Bai, Y., Fletcher, J., & Wilmut, I. (2010). Induced pluripotent stem cells: epigenetic memories and practical implications. Molecular Human Reproduction, 16, 880–885.
Ohi, Y., Qin, H., Hong, C., et al. (2011). Incomplete DNA methylation underlies a transcriptional memory of somatic cells in human iPS cells. Nature Cell Biology, 13, 541–549.
Xu, H., Yi, B. A., Wu, H., et al. (2011). Highly efficient derivation of ventricular cardiomyocytes from induced pluripotent stem cells with a distinct epigenetic signature. Cell Research, 22, 142–154.
Shao, K., Koch, C., Gupta, M. K., et al. (2012). Induced pluripotent mesenchymal stromal cell clones retain donor-derived differences in DNA methylation profiles. Molecular Therapy, 21, 240–250.
Rizzi, R., Di Pasquale, E., Portararo, P., et al. (2012). Post-natal cardiomyocytes can generate iPS cells with an enhanced capacity toward cardiomyogenic re-differentation. Cell Death and Differentiation, 19, 1162–1174.
Pfaff, N., Lachmann, N., Kohlscheen, S., et al. (2012). Efficient hematopoietic redifferentiation of induced pluripotent stem cells derived from primitive murine bone marrow cells. Stem Cells and Development, 21, 689–701.
Ghosh, Z., Wilson, K. D., Wu, Y., Hu, S., Quertermous, T., & Wu, J. C. (2010). Persistent donor cell gene expression among human induced pluripotent stem cells contributes to differences with human embryonic stem cells. PLoS One, 5, e8975.
Lee, S. B., Seo, D., Choi, D., et al. (2012). Contribution of hepatic lineage stage-specific donor memory to the differential potential of induced mouse pluripotent stem cells. Stem Cells, 30, 997–1007.
Sommer, C. A., Stadtfeld, M., Murphy, G. J., Hochedlinger, K., Kotton, D. N., & Mostoslavsky, G. (2009). Induced pluripotent stem cell generation using a single lentiviral stem cell cassette. Stem Cells, 27, 543–549.
Martins-Taylor, K., Nisler, B. S., Taapken, S. M., et al. (2011). Recurrent copy number variations in human induced pluripotent stem cells. Nature Biotechnology, 29, 488–491.
Muller, F. J., Schuldt, B. M., Williams, R., et al. (2011). A bioinformatic assay for pluripotency in human cells. Nature Methods, 8, 315–317.
Muller F.J., Brandl B., Loring J.F. (2008) Assessment of human pluripotent stem cells with PluriTest. In: StemBook. Cambridge, MA; 2008.
Acknowledgments
This work was funded by the State of Connecticut Stem Cell Seed Grants (#10SCA36 and #13-SCA-UCHC-11 to RMG) and the State of Connecticut Established Investigator Grant (#11SCB08 to HD). The authors are grateful for the stem cell services and technical support provided by Leann Crandall, Tiwanna Johnson and Jung Park from the University of Connecticut Stem Cell Core and Chromosome Facility We also acknowledge Dr. Judy Brown and Dr. Rachel O’Neil from the University of Connecticut Induced Pluripotent Stem Cell Core and Chromosome Facility for their expertise with chromosome analyses. The authors have no conflict of interest to declare.
Conflicts of Interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
This study was funded by the State of Connecticut Stem Cell Program/Department of Public Health. Contract grant numbers: #10SCA36 and #13-SCA-UCHC-11 (to RMG), #11SCB08 (to HD)
Rights and permissions
About this article
Cite this article
Guzzo, R.M., Scanlon, V., Sanjay, A. et al. Establishment of Human cell Type-Specific iPS cells with Enhanced Chondrogenic Potential. Stem Cell Rev and Rep 10, 820–829 (2014). https://doi.org/10.1007/s12015-014-9538-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-014-9538-8