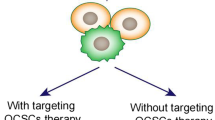
Abstract
Understanding the genetic and molecular mechanisms of ovarian cancer has been the focus of research efforts working toward the greater goal of improving cancer therapy for patients with residual disease after initial treatment with conventional surgery and neoadjuvant chemotherapy. The focus of this review will be centered on new therapeutic strategies based on Cancer Stem Cells studies of chemoresistant subpopulations, the prevention of metastasis, and individualized therapy in order to find the most successful combination of treatments to effectively treat human ovarian cancer. We reviewed recent literature (1993–2011) of novel treatment approaches to ovarian cancer stem cells. As the focus of ovarian cancer investigation has centered on the cancer stem cell model and the complexities that it presents in the development of effective treatments, the future of treating ovarian cancer lies in utilizing individualized treatment systems that include enhancing existing treatments, aiming for novel therapy targets, managing the plasticity of stem cells to induce cellular differentiation, and regulating oncogenic signaling pathways.


Similar content being viewed by others
References
National Cancer Institute: Surveillance epidemiology and End Results. http://seer.cancer.gov/statfacts/html/ovary.html.Retrieved December 18, 2010.
Ferlay, J., Parkin, D. M., & Steliarova-Foucher, E. (2008). Estimates of cancer incidence and mortality in Europe in. European Journal of Cancer, 46, 765–781.
European commission: Public Health. Available at: http://ec.europa.eu/health/ph_information/dissemination/diseases/cancer.htm. Retrieved December 15, 2010.
Leitao, M. M., Jr., & Chi, D. S. (2009). Surgical management of recurrent ovarian cancer. Seminars in Oncology, 36, 106–111.
Krasner, C., & Duska, L. (2009). Management of women with newly diagnosed ovarian cancer. Seminars in Oncology, 36, 91–105.
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J., & Clarke, M. F. (2003). Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America, 100, 3983–3988.
Collins, A. T., Berry, P. A., Hyde, C., Stower, M. J., & Maitland, N. J. (2005). Prospective identification of tumorigenic prostate cancer stem cells. Cancer Research, 65, 10946–10951.
Dalerba, P., Dylla, S. J., Park, I. K., et al. (2007). Phenotypic characterization of human colorectal cancer stem cells. Proceedings of the National Academy of Sciences of the United States of America, 104, 10158–10163.
Li, C., Heidt, D. G., Dalerba, P., et al. (2007). Identification of pancreatic cancer stem cells. Cancer Research, 67, 1030–1037.
Galli, R., Binda, E., Orfanelli, U., et al. (2004). Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Research, 64, 7011–7021.
Singh, S. K., Clarke, I. D., Terasaki, M., et al. (2003). Identification of a cancer stem cell in human brain tumors. Cancer Research, 63, 5821–5828.
Zhang, S., Balch, C., Chan, M. W., et al. (2008). Identification and characterization of ovarian cancer-initiating cells from primary human tumors. Cancer Research, 68, 4311–4320.
Baba, T., Convery, P. A., Matsumura, N., et al. (2009). Epigenetic regulation of CD133 and tumorigenicity of CD133+ ovarian cancer cells. Oncogene, 28, 209–218.
Curley, M. D., Therrien, V. A., Cummings, C. L., et al. (2009). CD133 expression defines a tumor initiating cell population in primary human ovarian cancer. Stem Cells, 27, 2875–2883.
Bapat, S. A., Mali, A. M., Koppikar, C. B., & Kurrey, N. K. (2005). Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Research, 65, 3025–3029.
Clarke, M. F., Dick, J. E., Dirks, P. B., et al. (2006). Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Research, 66, 9339–9344.
Ginestier, C., Hur, M. H., Charafe-Jauffret, E., et al. (2007). ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell, 1, 555–567.
Al-Hajj, M., & Clarke, M. F. (2004). Self-renewal and solid tumor stem cells. Oncogene, 23, 7274–7282.
Ruiz-Vela, A., Aguilar-Gallardo, C., & Simon, C. (2009). Building a framework for embryonic microenvironments and cancer stem cells. Stem Cell Reviews, 5, 319–327.
Vergote I, Trope CG, Amant F, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med 363:943–53.
Schwartz, P. E. (2002). Neoadjuvant chemotherapy for the management of ovarian cancer. Best Practice & Research. Clinical Obstetrics & Gynaecology, 16, 585–596.
Phillips, T. M., McBride, W. H., & Pajonk, F. (2006). The response of CD24(-/low)/CD44+ breast cancer-initiating cells to radiation. Journal of the National Cancer Institute, 98, 1777–1785.
Bao, S., Wu, Q., McLendon, R. E., et al. (2006). Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature, 444, 756–760.
Blagosklonny, M. V. (2007). Cancer stem cell and cancer stemloids: from biology to therapy. Cancer Biology & Therapy, 6, 1684–1690.
Ishii, H., Iwatsuki, M., Ieta, K., et al. (2008). Cancer stem cells and chemoradiation resistance. Cancer Science, 99, 1871–1877.
Hanahan, D., & Weinberg, R. A. (2011). Hallmarks of cancer: the next generation. Cell, 144, 646–674.
Gupta, P. B., Onder, T. T., Jiang, G., et al. (2009). Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell, 138, 645–659.
DeNardo, D. G., Andreu, P., & Coussens, L. M. (2010). Interactions between lymphocytes and myeloid cells regulate pro- versus anti-tumor immunity. Cancer Metastasis Reviews, 29, 309–316.
Grivennikov, S. I., Greten, F. R., & Karin, M. (2010). Immunity, inflammation, and cancer. Cell, 140, 883–899.
Qian, B. Z., & Pollard, J. W. (2010). Macrophage diversity enhances tumor progression and metastasis. Cell, 141, 39–51.
Karnoub, A. E., Dash, A. B., Vo, A. P., et al. (2007). Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature, 449, 557–563.
Lara, P. C., Lloret, M., Clavo, B., et al. (2009). Severe hypoxia induces chemo-resistance in clinical cervical tumors through MVP over-expression. Radiation Oncology, 4, 29.
Elloul, S., Vaksman, O., Stavnes, H. T., Trope, C. G., Davidson, B., & Reich, R. (2010). Mesenchymal-to-epithelial transition determinants as characteristics of ovarian carcinoma effusions. Clinical & Experimental Metastasis, 27, 161–172.
Pistollato, F., Abbadi, S., Rampazzo, E., et al. (2010). Intratumoral hypoxic gradient drives stem cells distribution and MGMT expression in glioblastoma. Stem cells Dayton, Ohio, 28, 851–862.
Greijer, A. E., van der Groep, P., Kemming, D., et al. (2005). Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1). The Journal of Pathology, 206, 291–304.
Levine, A.J., Puzio-Kuter, A.M. (2010) The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 3;330(6009):1340–4.
DeBerardinis, R. J. (2008). Is cancer a disease of abnormal cellular metabolism? New angles on an old idea. Genetics in Medicine, 10, 767–777.
Hildebrandt, M. A., Gu, J., & Wu, X. (2009). Pharmacogenomics of platinum-based chemotherapy in NSCLC. Expert Opinion on Drug Metabolism & Toxicology, 5, 745–755.
Surowiak, P., Materna, V., Kaplenko, I., et al. (2006). ABCC2 (MRP2, cMOAT) can be localized in the nuclear membrane of ovarian carcinomas and correlates with resistance to cisplatin and clinical outcome. Clinical Cancer Research, 12, 7149–7158.
Baekelandt, M. M., Holm, R., Nesland, J. M., Trope, C. G., & Kristensen, G. B. (2000). P-glycoprotein expression is a marker for chemotherapy resistance and prognosis in advanced ovarian cancer. Anticancer Research, 20, 1061–1067.
Lu, L., Katsaros, D., Wiley, A., Rigault de la Longrais, I. A., Puopolo, M., & Yu, H. (2007). Expression of MDR1 in epithelial ovarian cancer and its association with disease progression. Oncology Research, 16, 395–403.
van Herwaarden, A. E., Wagenaar, E., Karnekamp, B., Merino, G., Jonker, J. W., & Schinkel, A. H. (2006). Breast cancer resistance protein (Bcrp1/Abcg2) reduces systemic exposure of the dietary carcinogens aflatoxin B1, IQ and Trp-P-1 but also mediates their secretion into breast milk. Carcinogenesis, 27, 123–130.
Zhou, S., Schuetz, J. D., Bunting, K. D., et al. (2001). The ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem cells and is a molecular determinant of the side-population phenotype. Nature Medicine, 7, 1028–1034.
Alvi, A. J., Clayton, H., Joshi, C., et al. (2003). Functional and molecular characterisation of mammary side population cells. Breast Cancer Research, 5, R1–R8.
Cervello, I., Gil-Sanchis, C., Mas, A., et al. (2010). Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One, 5, e10964.
Hosonuma, S., Kobayashi, Y., Kojo, S., et al. (2011). Clinical significance of side population in ovarian cancer cells. Human Cell, 24, 9–12.
Szotek, P. P., Pieretti-Vanmarcke, R., Masiakos, P. T., et al. (2006). Ovarian cancer side population defines cells with stem cell-like characteristics and Mullerian Inhibiting Substance responsiveness. Proceedings of the National Academy of Sciences of the United States of America, 103, 11154–11159.
Hu, L., McArthur, C., & Jaffe, R. B. (2010). Ovarian cancer stem-like side-population cells are tumourigenic and chemoresistant. British Journal of Cancer, 102, 1276–1283.
Jones, R. J., Matsui, W. H., & Smith, B. D. (2004). Cancer stem cells: are we missing the target? Journal of the National Cancer Institute, 96, 583–585.
Burger, H., Loos, W. J., Eechoute, K., Verweij, J., Mathijssen, R. H. J., & Wiemer, E. A. C. (2011). Drug transporters of platinum-based anticancer agents and their clinical significance. Drug Resistance Updates, 14, 22–34.
Kamazawa, S., Kigawa, J., Kanamori, Y., et al. (2002). Multidrug resistance gene-1 is a useful predictor of Paclitaxel-based chemotherapy for patients with ovarian cancer. Gynecologic Oncology, 86, 171–176.
Yang, G. F., He, W. P., Cai, M. Y., et al. (2010). Intensive expression of Bmi-1 is a new independent predictor of poor outcome in patients with ovarian carcinoma. BMC Cancer, 10, 133.
Wang, E., Bhattacharyya, S., Szabolcs, A., et al. (2011). Enhancing chemotherapy response with Bmi-1 silencing in ovarian cancer. PLoS One, 6, e17918.
Rodriguez-Antona, C. (2010). Pharmacogenomics of paclitaxel. Pharmacogenomics, 11, 621–623.
Wicha, M. S., Liu, S., & Dontu, G. (2006). Cancer stem cells: an old idea–a paradigm shift. Cancer Research, 66, 1883–1890. discussion 95-6.
Sell, S., & Pierce, G. B. (1994). Maturation arrest of stem cell differentiation is a common pathway for the cellular origin of teratocarcinomas and epithelial cancers. Laboratory Investigation, 70, 6–22.
Cavenee, W. K., Dryja, T. P., Phillips, R. A., et al. (1983). Expression of recessive alleles by chromosomal mechanisms in retinoblastoma. Nature, 305, 779–784.
Nowell, P. C. (1993). Foundations in cancer research. Chromosomes and cancer: the evolution of an idea. Advances in Cancer Research, 62, 1–17.
Wallace-Brodeur, R. R., & Lowe, S. W. (1999). Clinical implications of p53 mutations. Cellular and Molecular Life Sciences, 55, 64–75.
Herr, I., & Debatin, K. M. (2001). Cellular stress response and apoptosis in cancer therapy. Blood, 98, 2603–2614.
Reed, E. C. (1999). Cancer Chemotherapy and Biological Response Modifiers, 18, 144–151.
Rolitsky, C. D., Theil, K. S., McGaughy, V. R., Copeland, L. J., & Niemann, T. H. (1999). HER-2/neu amplification and overexpression in endometrial carcinoma. International Journal of Gynecological Pathology, 18, 138–143.
Slamon, D. J., Godolphin, W., Jones, L. A., et al. (1989). Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science, 244, 707–712.
Kim, J. W., Lee, C. G., Lyu, M. S., et al. (1997). A new cell line from human undifferentiated carcinoma of the ovary: establishment and characterization. Journal of Cancer Research and Clinical Oncology, 123, 82–90.
Perez-Caro, M., Cobaleda, C., Gonzalez-Herrero, I., et al. (2009). Cancer induction by restriction of oncogene expression to the stem cell compartment. EMBO Journal, 28, 8–20.
Bapat, S. A. (2010). Human ovarian cancer stem cells. Reproduction, 140, 33–41.
Lawrenson, K., & Gayther, S. A. (2009). Ovarian cancer: a clinical challenge that needs some basic answers. PLoS Medicine, 6, e25.
Deonarain, M. P., Kousparou, C. A., & Epenetos, A. A. (2009). Antibodies targeting cancer stem cells: a new paradigm in immunotherapy? MAbs, 1, 12–25.
Ferrandina, G., Bonanno, G., Pierelli, L., et al. (2008). Expression of CD133-1 and CD133-2 in ovarian cancer. International Journal of Gynecological Cancer, 18, 506–514.
Smith, L. M., Nesterova, A., Ryan, M. C., et al. (2008). CD133/prominin-1 is a potential therapeutic target for antibody-drug conjugates in hepatocellular and gastric cancers. British Journal of Cancer, 99, 100–109.
Naor, D., Sionov, R. V., & Ish-Shalom, D. (1997). CD44: structure, function, and association with the malignant process. Advances in Cancer Research, 71, 241–319.
Steffensen, K. D., Alvero, A. B., Yang, Y., et al. (2011). Prevalence of epithelial ovarian cancer stem cells correlates with recurrence in early-stage ovarian cancer. J Oncol, 2011, 620523.
Alvero, A. B., Chen, R., Fu, H. H., et al. (2009). Molecular phenotyping of human ovarian cancer stem cells unravels the mechanisms for repair and chemoresistance. Cell Cycle, 8, 158–166.
Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer 46:1271–7.
Heider, K. H., Kuthan, H., Stehle, G., & Munzert, G. (2004). CD44v6: a target for antibody-based cancer therapy. Cancer Immunology, Immunotherapy, 53, 567–579.
De Stefano I, Battaglia A, Zannoni GF, et al. Hyaluronic acid-paclitaxel: effects of intraperitoneal administration against CD44(+) human ovarian cancer xenografts. Cancer Chemother Pharmacol.
Visvader, J. E., & Lindeman, G. J. (2008). Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nature Reviews. Cancer, 8, 755–768.
Bellone, S., Siegel, E. R., Cocco, E., et al. (2009). Overexpression of epithelial cell adhesion molecule in primary, metastatic, and recurrent/chemotherapy-resistant epithelial ovarian cancer: implications for epithelial cell adhesion molecule-specific immunotherapy. International Journal of Gynecological Cancer, 19, 860–866.
Fields, A. L., Keller, A., Schwartzberg, L., et al. (2009). Adjuvant therapy with the monoclonal antibody Edrecolomab plus fluorouracil-based therapy does not improve overall survival of patients with stage III colon cancer. Journal of Clinical Oncology, 27, 1941–1947.
Xiang, W., Wimberger, P., Dreier, T., et al. (2003). Cytotoxic activity of novel human monoclonal antibody MT201 against primary ovarian tumor cells. Journal of Cancer Research and Clinical Oncology, 129, 341–348.
Richter CE, Cocco E, Bellone S, et al. High-grade, chemotherapy-resistant ovarian carcinomas overexpress epithelial cell adhesion molecule (EpCAM) and are highly sensitive to immunotherapy with MT201, a fully human monoclonal anti-EpCAM antibody. Am J Obstet Gynecol 203:582 e1–7.
Fletcher, J. I., Haber, M., Henderson, M. J., & Norris, M. D. (2010). ABC transporters in cancer: more than just drug efflux pumps. Nature Reviews. Cancer, 10, 147–156.
Schatton, T., Frank, N. Y., & Frank, M. H. (2009). Identification and targeting of cancer stem cells. Bioessays, 31, 1038–1049.
Soignet, S. L., Benedetti, F., Fleischauer, A., et al. (1998). Clinical study of 9-cis retinoic acid (LGD1057) in acute promyelocytic leukemia. Leukemia, 12(10), 1518–1521.
Sell, S. (2004). Stem cell origin of cancer and differentiation therapy. Critical Reviews in Oncology/Hematology, 51, 1–28.
Stefansson, O. A., & Esteller, M. (2011). EZH2-mediated epigenetic repression of DNA repair in promoting breast tumor initiating cells. Breast Cancer Research, 13, 309.
Chang, C. J., Yang, J. Y., Xia, W., et al. (2011). EZH2 promotes expansion of breast tumor initiating cells through activation of RAF1-beta-catenin signaling. Cancer Cell, 19, 86–100.
Taddei, A., Roche, D., Bickmore, W. A., & Almouzni, G. (2005). The effects of histone deacetylase inhibitors on heterochromatin: implications for anticancer therapy? EMBO Reports, 6, 520–524.
Bergmann, M., Romirer, I., Sachet, M., et al. (2001). A genetically engineered influenza A virus with ras-dependent oncolytic properties. Cancer Research, 61, 8188–8193.
Behbod, F., & Rosen, J. M. (2005). Will cancer stem cells provide new therapeutic targets? Carcinogenesis, 26, 703–711.
Blagosklonny, M. V. (2005). Teratogens as anti-cancer drugs. Cell Cycle, 4, 1518–1521.
Taipale, J., Chen, J. K., Cooper, M. K., et al. (2000). Effects of oncogenic mutations in Smoothened and Patched can be reversed by cyclopamine. Nature, 406, 1005–1009.
Peacock, C. D., Wang, Q., Gesell, G. S., et al. (2007). Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proceedings of the National Academy of Sciences of the United States of America, 104, 4048–4053.
Banker, D. E., Mayer, S. J., Li, H. Y., Willman, C. L., Appelbaum, F. R., & Zager, R. A. (2004). Cholesterol synthesis and import contribute to protective cholesterol increments in acute myeloid leukemia cells. Blood, 104, 1816–1824.
Stirewalt, D. L., Appelbaum, F. R., Willman, C. L., Zager, R. A., & Banker, D. E. (2003). Mevastatin can increase toxicity in primary AMLs exposed to standard therapeutic agents, but statin efficacy is not simply associated with ras hotspot mutations or overexpression. Leukemia Research, 27, 133–145.
Wu, J., Wong, W. W., Khosravi, F., Minden, M. D., & Penn, L. Z. (2004). Blocking the Raf/MEK/ERK pathway sensitizes acute myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer Research, 64, 6461–6468.
Martelli, A. M., Nyakern, M., Tabellini, G., et al. (2006). Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia, 20, 911–928.
Krause, D. S., & Van Etten, R. A. (2007). Right on target: eradicating leukemic stem cells. Trends in Molecular Medicine, 13, 470–481.
Kersten, S., & Wahli, W. (2000). Peroxisome proliferator activated receptor agonists. EXS, 89, 141–151.
Grommes, C., Landreth, G. E., & Heneka, M. T. (2004). Antineoplastic effects of peroxisome proliferator-activated receptor gamma agonists. The Lancet Oncology, 5, 419–429.
Koeffler, H. P. (2003). Peroxisome proliferator-activated receptor gamma and cancers. Clinical Cancer Research, 9, 1–9.
Kopelovich, L., Fay, J. R., Glazer, R. I., & Crowell, J. A. (2002). Peroxisome proliferator-activated receptor modulators as potential chemopreventive agents. Molecular Cancer Therapeutics, 1, 357–363.
Tontonoz, P., Singer, S., Forman, B. M., et al. (1997). Terminal differentiation of human liposarcoma cells induced by ligands for peroxisome proliferator-activated receptor gamma and the retinoid X receptor. Proceedings of the National Academy of Sciences of the United States of America, 94, 237–241.
Sarraf, P., Mueller, E., Jones, D., et al. (1998). Differentiation and reversal of malignant changes in colon cancer through PPARgamma. Nature Medicine, 4, 1046–1052.
Morrison, S. J., Wandycz, A. M., Hemmati, H. D., Wright, D. E., & Weissman, I. L. (1997). Identification of a lineage of multipotent hematopoietic progenitors. Development, 124, 1929–1939.
Ruiz-Vela, A., Aguilar-Gallardo, C., Martinez-Arroyo, A.M., Soriano-Navarro, M., Ruiz, V., Simon, C. (2011). Specific unsaturated fatty acids enforce the transdifferentiation of human cancer cells toward adipocyte-like cells. Stem Cell Rev.
Semple, R. K., Chatterjee, V. K., & O’Rahilly, S. (2006). PPAR gamma and human metabolic disease. The Journal of Clinical Investigation, 116, 581–589.
Vignati, S., Albertini, V., Rinaldi, A., et al. (2006). Cellular and molecular consequences of peroxisome proliferator-activated receptor-gamma activation in ovarian cancer cells. Neoplasia, 8, 851–861.
Holland, C. M., Saidi, S. A., Evans, A. L., et al. (2004). Transcriptome analysis of endometrial cancer identifies peroxisome proliferator-activated receptors as potential therapeutic targets. Molecular Cancer Therapeutics, 3, 993–1001.
Shigeto, T., Yokoyama, Y., Xin, B., & Mizunuma, H. (2007). Peroxisome proliferator-activated receptor alpha and gamma ligands inhibit the growth of human ovarian cancer. Oncology Reports, 18, 833–840.
Xin, B., Yokoyama, Y., Shigeto, T., Futagami, M., & Mizunuma, H. (2007). Inhibitory effect of meloxicam, a selective cyclooxygenase-2 inhibitor, and ciglitazone, a peroxisome proliferator-activated receptor gamma ligand, on the growth of human ovarian cancers. Cancer, 110, 791–800.
Yang, Y. C., Tsao, Y. P., Ho, T. C., & Choung, I. P. (2007). Peroxisome proliferator-activated receptor-gamma agonists cause growth arrest and apoptosis in human ovarian carcinoma cell lines. International Journal of Gynecological Cancer, 17, 418–425.
Gotlieb, W. H., Saumet, J., Beauchamp, M. C., et al. (2008). In vitro metformin anti-neoplastic activity in epithelial ovarian cancer. Gynecologic Oncology, 110, 246–250.
Rattan, R., Graham, R. P., Maguire, J. L., Giri, S., & Shridhar, V. (2011). Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia, 13, 483–491.
Hirsch, H. A., Iliopoulos, D., Tsichlis, P. N., & Struhl, K. (2009). Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Research, 69, 7507–7511.
Davis, M. E., Chen, Z. G., & Shin, D. M. (2008). Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Reviews. Drug Discovery, 7, 771–782.
Chen, Z. G. (2010). Small-molecule delivery by nanoparticles for anticancer therapy. Trends in Molecular Medicine, 16, 594–602.
Sutton, D., Nasongkla, N., Blanco, E., & Gao, J. (2007). Functionalized micellar systems for cancer targeted drug delivery. Pharmaceutical Research, 24, 1029–1046.
Boddy, A. V., Plummer, E. R., Todd, R., et al. (2005). A phase I and pharmacokinetic study of paclitaxel poliglumex (XYOTAX), investigating both 3-weekly and 2-weekly schedules. Clinical Cancer Research, 11, 7834–7840.
Sabbatini, P., Aghajanian, C., Dizon, D., et al. (2004). Phase II study of CT-2103 in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. Journal of Clinical Oncology, 22, 4523–4531.
Eldar-Boock, A., Miller, K., Sanchis, J., Lupu, R., Vicent, M. J., & Satchi-Fainaro, R. (2011). Integrin-assisted drug delivery of nano-scaled polymer therapeutics bearing paclitaxel. Biomaterials, 32, 3862–3874.
Folkman, J. (2007). Angiogenesis: an organizing principle for drug discovery? Nature Reviews. Drug Discovery, 6, 273–286.
Leskela, S., Leandro-Garcia, L. J., Mendiola, M., et al. (2011). The miR-200 family controls beta-tubulin III expression and is associated with paclitaxel-based treatment response and progression-free survival in ovarian cancer patients. Endocrine-Related Cancer, 18, 85–95.
Korpal, M., Lee, E. S., Hu, G., & Kang, Y. (2008). The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. Journal of Biological Chemistry, 283, 14910–14914.
Hu, X., Macdonald, D. M., Huettner, P. C., et al. (2009). A miR-200 microRNA cluster as prognostic marker in advanced ovarian cancer. Gynecologic Oncology, 114, 457–464.
Wu, Q., Guo, R., Lin, M., Zhou, B., & Wang, Y. (2011). MicroRNA-200a inhibits CD133/1+ ovarian cancer stem cells migration and invasion by targeting E-cadherin repressor ZEB2. Gynecologic Oncology, 122, 149–154.
Bhattacharya, R., Nicoloso, M., Arvizo, R., et al. (2009). MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer. Cancer Research, 69, 9090–9095.
Bell, D. A. (2005). Origins and molecular pathology of ovarian cancer. Modern Pathology, 18(Suppl 2), S19–S32.
Saga, Y., Ohwada, M., Suzuki, M., et al. (2008). Glutathione peroxidase 3 is a candidate mechanism of anticancer drug resistance of ovarian clear cell adenocarcinoma. Oncology Reports, 20, 1299–1303.
Bast, R. C., Jr., Hennessy, B., & Mills, G. B. (2009). The biology of ovarian cancer: new opportunities for translation. Nature Reviews. Cancer, 9, 415–428.
Frumovitz, M., Schmeler, K. M., Malpica, A., Sood, A. K., & Gershenson, D. M. (2010). Unmasking the complexities of mucinous ovarian carcinoma. Gynecologic Oncology, 117, 491–496.
Gilks, C. B., & Prat, J. (2009). Ovarian carcinoma pathology and genetics: recent advances. Human Pathology, 40, 1213–1223.
Storey, D. J., Rush, R., Stewart, M., et al. (2008). Endometrioid epithelial ovarian cancer: 20 years of prospectively collected data from a single center. Cancer, 112, 2211–2220.
Auersperg, N., Wong, A. S., Choi, K. C., Kang, S. K., & Leung, P. C. (2001). Ovarian surface epithelium: biology, endocrinology, and pathology. Endocrine Reviews, 22, 255–288.
O’Brien, C. A., Pollett, A., Gallinger, S., & Dick, J. E. (2007). A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature, 445, 106–110.
Yin, S., Li, J., Hu, C., et al. (2007). CD133 positive hepatocellular carcinoma cells possess high capacity for tumorigenicity. International Journal of Cancer, 120, 1444–1450.
Rappa, G., Fodstad, O., & Lorico, A. (2008). The stem cell-associated antigen CD133 (Prominin-1) is a molecular therapeutic target for metastatic melanoma. Stem Cells, 26, 3008–3017.
Angelastro, J. M., & Lame, M. W. (2010). Overexpression of CD133 promotes drug resistance in C6 glioma cells. Molecular Cancer Research, 8, 1105–1115.
Saigusa, S., Tanaka, K., Toiyama, Y., et al. (2010). Immunohistochemical features of CD133 expression: association with resistance to chemoradiotherapy in rectal cancer. Oncology Reports, 24, 345–350.
Afify, A. M., Ferguson, A. W., Davila, R. M., & Werness, B. A. (2001). Expression of CD44S and CD44v5 is more common in stage III than in stage I serous ovarian carcinomas. Applied Immunohistochemistry & Molecular Morphology, 9, 309–314.
Sillanpaa, S., Anttila, M. A., Voutilainen, K., et al. (2003). CD44 expression indicates favorable prognosis in epithelial ovarian cancer. Clinical Cancer Research, 9, 5318–5324.
Hong, S. P., Wen, J., Bang, S., Park, S., & Song, S. Y. (2009). CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. International Journal of Cancer, 125, 2323–2331.
Horst, D., Kriegl, L., Engel, J., Kirchner, T., & Jung, A. (2009). Prognostic significance of the cancer stem cell markers CD133, CD44, and CD166 in colorectal cancer. Cancer Investigation, 27, 844–850.
Takaishi, S., Okumura, T., Tu, S., et al. (2009). Identification of gastric cancer stem cells using the cell surface marker CD44. Stem Cells, 27, 1006–1020.
Oliveras-Ferraros, C., Vazquez-Martin, A., Martin-Castillo, B., et al. (2010). Dynamic emergence of the mesenchymal CD44(pos)CD24(neg/low) phenotype in HER2-gene amplified breast cancer cells with de novo resistance to trastuzumab (Herceptin). Biochemical and Biophysical Research Communications, 397, 27–33.
Sviatoha, V., Tani, E., Kleina, R., Sperga, M., & Skoog, L. (2010). Immunohistochemical analysis of the S100A1, S100B, CD44 and Bcl-2 antigens and the rate of cell proliferation assessed by Ki-67 antibody in benign and malignant melanocytic tumours. Melanoma Research, 20, 118–125.
Abeysinghe, H. R., Cao, Q., Xu, J., et al. (2003). THY1 expression is associated with tumor suppression of human ovarian cancer. Cancer Genetics and Cytogenetics, 143, 125–132.
Lung, H. L., Bangarusamy, D. K., Xie, D., et al. (2005). THY1 is a candidate tumour suppressor gene with decreased expression in metastatic nasopharyngeal carcinoma. Oncogene, 24, 6525–6532.
Yang, Z. F., Ho, D. W., Ng, M. N., et al. (2008). Significance of CD90+ cancer stem cells in human liver cancer. Cancer Cell, 13, 153–166.
Kristiansen, G., Denkert, C., Schluns, K., Dahl, E., Pilarsky, C., & Hauptmann, S. (2002). CD24 is expressed in ovarian cancer and is a new independent prognostic marker of patient survival. American Journal of Pathology, 161, 1215–1221.
Lee, H. J., Kim, D. I., Kwak, C., Ku, J. H., & Moon, K. C. (2008). Expression of CD24 in clear cell renal cell carcinoma and its prognostic significance. Urology, 72, 603–607.
Nagy, B., Szendroi, A., & Romics, I. (2009). Overexpression of CD24, c-myc and phospholipase 2A in prostate cancer tissue samples obtained by needle biopsy. Pathology Oncology Research, 15, 279–283.
Yang, X. R., Xu, Y., Yu, B., et al. (2009). CD24 is a novel predictor for poor prognosis of hepatocellular carcinoma after surgery. Clinical Cancer Research, 15, 5518–5527.
Kajiyama, H., Shibata, K., Ino, K., Mizutani, S., Nawa, A., & Kikkawa, F. (2010). The expression of dipeptidyl peptidase IV (DPPIV/CD26) is associated with enhanced chemosensitivity to paclitaxel in epithelial ovarian carcinoma cells. Cancer Science, 101, 347–354.
Pang, R., Law, W. L., Chu, A. C., et al. (2010). A subpopulation of CD26+ cancer stem cells with metastatic capacity in human colorectal cancer. Cell Stem Cell, 6, 603–615.
Wei, H., Wang, C., & Chen, L. (2006). Proliferating cell nuclear antigen, survivin, and CD34 expressions in pancreatic cancer and their correlation with hypoxia-inducible factor 1alpha. Pancreas, 32, 159–163.
Shin, S. J., Jeung, H. C., Ahn, J. B., et al. (2008). Mobilized CD34+ cells as a biomarker candidate for the efficacy of combined maximal tolerance dose and continuous infusional chemotherapy and G-CSF surge in gastric cancer. Cancer Letters, 270, 269–276.
Jiang, X., Forrest, D., Nicolini, F., et al. (2010). Properties of CD34+ CML stem/progenitor cells that correlate with different clinical responses to imatinib mesylate. Blood, 116, 2112–2121.
Liu, L., Chen, R., Huang, S., et al. (2010). Knockdown of SOD1 sensitizes the CD34+ CML cells to imatinib therapy. Med Oncol.
Salnikov, A. V., Groth, A., Apel, A., et al. (2009). Targeting of cancer stem cell marker EpCAM by bispecific antibody EpCAMxCD3 inhibits pancreatic carcinoma. Journal of Cellular and Molecular Medicine, 13, 4023–4033.
Yamashita, T., Ji, J., Budhu, A., et al. (2009). EpCAM-positive hepatocellular carcinoma cells are tumor-initiating cells with stem/progenitor cell features. Gastroenterology, 136, 1012–1024.
Terris, B., Cavard, C., & Perret, C. (2010). EpCAM, a new marker for cancer stem cells in hepatocellular carcinoma. Journal of Hepatology, 52, 280–281.
Colvin, M., Russo, J. E., Hilton, J., Dulik, D. M., & Fenselau, C. (1988). Enzymatic mechanisms of resistance to alkylating agents in tumor cells and normal tissues. Advances in Enzyme Regulation, 27, 211–221.
Eastman, A., & Schulte, N. (1988). Enhanced DNA repair as a mechanism of resistance to cis-diamminedichloroplatinum(II). Biochemistry, 27, 4730–4734.
Bunting, K. D., Lindahl, R., & Townsend, A. J. (1994). Oxazaphosphorine-specific resistance in human MCF-7 breast carcinoma cell lines expressing transfected rat class 3 aldehyde dehydrogenase. Journal of Biological Chemistry, 269, 23197–23203.
Carpentino, J. E., Hynes, M. J., Appelman, H. D., et al. (2009). Aldehyde dehydrogenase-expressing colon stem cells contribute to tumorigenesis in the transition from colitis to cancer. Cancer Research, 69, 8208–8215.
Chen, Y. C., Chen, Y. W., Hsu, H. S., et al. (2009). Aldehyde dehydrogenase 1 is a putative marker for cancer stem cells in head and neck squamous cancer. Biochemical and Biophysical Research Communications, 385, 307–313.
Morimoto, K., Kim, S. J., Tanei, T., et al. (2009). Stem cell marker aldehyde dehydrogenase 1-positive breast cancers are characterized by negative estrogen receptor, positive human epidermal growth factor receptor type 2, and high Ki67 expression. Cancer Science, 100, 1062–1068.
Li, T., Su, Y., Mei, Y., et al. (2010). ALDH1A1 is a marker for malignant prostate stem cells and predictor of prostate cancer patients’ outcome. Laboratory Investigation, 90, 234–244.
Bleau, A. M., Huse, J. T., & Holland, E. C. (2009). The ABCG2 resistance network of glioblastoma. Cell Cycle, 8, 2936–2944.
Hegedus, C., Ozvegy-Laczka, C., Apati, A., et al. (2009). Interaction of nilotinib, dasatinib and bosutinib with ABCB1 and ABCG2: implications for altered anti-cancer effects and pharmacological properties. British Journal of Pharmacology, 158, 1153–1164.
Agarwal, S., Sane, R., Gallardo, J. L., Ohlfest, J. R., & Elmquist, W. F. (2010). Distribution of gefitinib to the brain is limited by P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2)-mediated active efflux. Journal of Pharmacology and Experimental Therapeutics, 334, 147–155.
Ding, X. W., Wu, J. H., & Jiang, C. P. (2010). ABCG2: a potential marker of stem cells and novel target in stem cell and cancer therapy. Life Sciences, 86, 631–637.
Disclosures
The authors indicate no potential conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Cristóbal Aguilar-Gallardo, Emily Cecilia Rutledge and Ana M. Martínez-Arroyo have contributed equally to this work
Rights and permissions
About this article
Cite this article
Aguilar-Gallardo, C., Rutledge, E.C., Martínez-Arroyo, A.M. et al. Overcoming Challenges of Ovarian Cancer Stem Cells: Novel Therapeutic Approaches. Stem Cell Rev and Rep 8, 994–1010 (2012). https://doi.org/10.1007/s12015-011-9344-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-011-9344-5