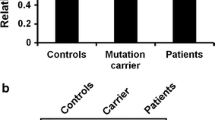
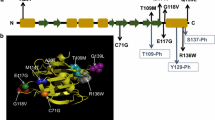
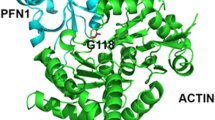
Abstract
Parkinson’s disease (PD) is one of the most common neurodegenerative diseases. Genes which have been implicated in autosomal-recessive PD include PARK2 which codes for parkin, an E3 ubiquitin ligase that participates in a variety of cellular activities. In this study, we compared parkin-mutant primary fibroblasts, from a patient with parkin compound heterozygous mutations, to healthy control cells. Western blot analysis of proteins obtained from patient’s fibroblasts showed quantitative differences of many proteins involved in the cytoskeleton organization with respect to control cells. These molecular alterations are accompanied by changes in the organization of actin stress fibers and biomechanical properties, as revealed by confocal laser scanning microscopy and atomic force microscopy. In particular, parkin deficiency is associated with a significant increase of Young’s modulus of null-cells in comparison to normal fibroblasts. The current study proposes that parkin influences the spatial organization of actin filaments, the shape of human fibroblasts, and their elastic response to an external applied force.




Similar content being viewed by others
Abbreviations
- α-Tub:
-
α-Tubulin
- AFM:
-
Atomic force microscopy
- CLSM:
-
Confocal laser scanning microscopy
- ABPs:
-
Actin-binding proteins
- COF:
-
Cofilin
- F-actin:
-
Filamentous actin
- Gsk-3:
-
Glycogen synthase kinase-3
- PD:
-
Parkinson’s disease
- CTR:
-
Healthy control
- P1:
-
PD patient
- siRNAs:
-
Interfering RNAs
- LIMK:
-
LIM kinase
- MLC:
-
Myosin light chain
- p-COF:
-
Phosphorylated cofilin
- Akt:
-
Protein kinase B
- PAK:
-
Rac-Cdc42 p21-activated kinase
- ROI:
-
Region of interest
- ROCK:
-
Rho associated kinase
References
Klein, C., & Westenberger, A. (2012). Genetics of Parkinson’s disease. Cold Spring Harbor Perspectives in Medicine, 2(1), a008888. doi:10.1101/cshperspect.a008888.
Kahle, P. J., & Haass, C. (2004). How does parkin ligate ubiquitin to Parkinson’s disease? EMBO Reports, 5(7), 681–685. doi:10.1038/sj.embor.7400188.
Pacelli, C., De Rasmo, D., Signorile, A., Grattagliano, I., di Tullio, G., D’Orazio, A., et al. (2011). Mitochondrial defect and PGC-1α dysfunction in parkin-associated familial Parkinson’s disease. Biochimica et Biophysica Acta, 1812(8), 1041–1053. doi:10.1016/j.bbadis.2010.12.022.
Winklhofer, K. F. (2014). Parkin and mitochondrial quality control: Toward assembling the puzzle. Trends in Cell Biology, 24(6), 332–334. doi:10.1016/j.tcb.2014.01.001.
Zanon, A., Rakovic, A., Blankenburg, H., Doncheva, N. T., Schwienbacher, C., Serafin, A., et al. (2013). Profiling of Parkin-binding partners using tandem affinity purification. PLoS ONE, 8(11), 78648. doi:10.1371/journal.pone.0078648.
Sarraf, S. A., Raman, M., Guarani-Pereira, V., Sowa, M. E., Huttlin, E. L., Gygi, S. P., et al. (2013). Landscape of the PARKIN-dependent ubiquitylome in response to mitochondrial depolarization. Nature, 496(7445), 372–376. doi:10.1038/nature12043.
Gillardon, F. (2009). Leucine-rich repeat kinase 2 phosphorylates brain tubulin-beta isoforms and modulates microtubule stability a point of convergence in parkinsonian neurodegeneration? Journal of Neurochemistry, 110(5), 1514–1522. doi:10.1111/j.1471-4159.2009.06235.
Häbig, K., Gellhaar, S., Heim, B., Djuric, V., Giesert, F., Wurst, W., et al. (2013). LRRK2 guides the actin cytoskeleton at growth cones together with ARHGEF7 and tropomyosin 4. Biochimica et Biophysica Acta, 1832(12), 2352–2367. doi:10.1016/j.bbadis.2013.09.009.
Cartelli, D., Goldwurm, S., Casagrande, F., Pezzoli, G., & Cappelletti, G. (2012). Microtubule destabilization is shared by genetic and idiopathic Parkinson’s disease patient fibroblasts. PLoS ONE, 7(5), e37467. doi:10.1371/journal.pone.0037467.
Kubo, S. I., Kitami, T., Noda, S., Shimura, H., Uchiyama, Y., Asakawa, S., et al. (2001). Parkin is associated with cellular vesicles. Journal of Neurochemistry, 78(1), 42–54. doi:10.1046/j.1471-4159.2001.00364.x.
Revenu, C., Athman, R., Robine, S., & Louvard, D. (2004). The co-workers of actin filaments: from cell structures to signals. Nature Reviews Molecular Cell Biology, 8, 635–646. doi:10.1038/nrm1437.
Vergara, D., Simeone, P., del Boccio, P., Toto, C., Pieragostino, D., Tinelli, A., et al. (2013). Comparative proteome profiling of breast tumor cell lines by gel electrophoresis and mass spectrometry reveals an epithelial mesenchymal transition associated protein signature. Molecular BioSystems, 6, 1127–1138. doi:10.1039/c2mb25401h.
De Masi, R., Vergara, D., Pasca, S., Acierno, R., Greco, M., Spagnolo, L., et al. (2009). PBMCs protein expression profile in relapsing IFN-treated multiple sclerosis: A pilot study on relation to clinical findings and brain atrophy. Journal of Neuroimmunology, 210(1–2), 80–86. doi:10.1016/j.jneuroim.2009.03.002.
Uribe, R., & Jay, D. (2009). A review of actin binding proteins: New perspectives. Molecular Biology Reports, 36(1), 121–125. doi:10.1007/s11033-007-9159-2.
Dent, E. W., & Gertler, F. B. (2003). Cytoskeletal dynamics and transport in growth cone motility and axon guidance. Neuron, 40(2), 209–227. doi:10.1016/S0896-6273(03)00633-0.
Sarmiere, P. D., & Bamburg, J. R. (2004). Regulation of the neuronal actin cytoskeleton by ADF/cofilin. Journal of Neurobiology, 58(1), 103–117. doi:10.1002/neu.10267.
Schonhofen, P., de Medeiros, L. M., Chatain, C. P., Bristot, I. J., & Klamt, F. (2014). Cofilin/actin rod formation by dysregulation of cofilin-1 activity as a central initial step in neurodegeneration. Mini Review in Medicinal Chemistry, 14(5), 393–400. doi:10.2174/1389557514666140506161458.
Mizuno, K. (2013). Signaling mechanisms and functional roles of cofilin phosphorylation and dephosphorylation. Cellular Signalling, 25(2), 457–469. doi:10.1016/j.cellsig.2012.11.001.
Riento, K., & Ridley, A. J. (2003). Rocks: Multifunctional kinases in cell behaviour. Nature Reviews Molecular Cell Biology, 4(6), 446–456. doi:10.1038/nrm1128.
Ferretta, A., Gaballo, A., Tanzarella, P., Piccoli, C., Capitanio, N., Nico, B., et al. (2014). Effect of resveratrol on mitochondrial function: Implications in parkin-associated familiar parkinson’s disease. Biochimica et Biophysica Acta, 1842(7), 902–915. doi:10.1016/j.bbadis.2014.02.010.
Boudaoud, A., Burian, A., Borowska-Wykręt, D., Uyttewaal, M., Wrzalik, R., Kwiatkowska, D., et al. (2014). FibrilTool, an ImageJ plug-into quantify fibrillar structures in raw microscopy images. Nature Protocols, 9, 457–463. doi:10.1038/nprot.2014.024.
Leporatti, S., Vergara, D., Zacheo, A., Vergaro, V., Maruccio, G., Cingolani, R., et al. (2009). Cytomechanical and topological investigation of MCF-7 cells by scanning force microscopy. Nanotechnology, 20(5), 55103–55108. doi:10.1088/0957-4484/20/5/055103.
Vergara, D., Martignago, R., Leporatti, S., Bonsegna, S., Maruccio, G., De Nuccio, F., et al. (2009). Biomechanical and proteomic analysis of INF- beta-treated astrocytes. Nanotechnology, 20(45), 455106–455115. doi:10.1088/0957-4484/20/45/455106.
Butt, H. J., & Jaschke, M. (1995). Calculation of thermal noise in atomic force microscopy. Nanotechnology, 6, 1–7. doi:10.1088/0957-4484/6/1/001.
Takahashi-Yanaga, F., & Sasaguri, T. (2008). GSK-3beta regulates cyclin D1 expression: A new target for chemotherapy. Cellular Signalling, 4, 581–589. doi:10.1016/j.cellsig.2007.10.018.
Rawal, N., Corti, O., Sacchetti, P., Ardilla-Osorio, H., Sehat, B., Brice, A., et al. (2009). Parkin protects dopaminergic neurons from excessive Wnt/beta-catenin signaling. Biochemical and Biophysical Research Communications, 388(3), 473–478. doi:10.1016/j.bbrc.2009.07.014.
Sousa, V. L., Bellani, S., Giannandrea, M., Yousuf, M., Valtorta, F., Meldolesi, J., et al. (2009). {alpha}-synuclein and its A30P mutant affect actin cytoskeletal structure and dynamics. Molecular Biology of the Cell, 16, 3725–3739. doi:10.1091/mbc.E08-03-0302.
Esposito, A., Dohm, C. P., Kermer, P., Bähr, M., & Wouters, F. S. (2007). Alpha-synuclein and its disease-related mutants interact differentially with the microtubule protein tau and associate with the actin cytoskeleton. Neurobiology of Diseases, 3, 521–531. doi:10.1016/j.nbd.2007.01.014.
Kim, K. H., & Son, J. H. (2010). PINK1 gene knockdown leads to increased binding of parkin with actin filament. Neuroscience Letters, 468(3), 272–276. doi:10.1016/j.neulet.2009.11.011.
Lim, M. K., Kawamura, T., Ohsawa, Y., Ohtsubo, M., Asakawa, S., Takayanagi, A., et al. (2007). Parkin interacts with LIM Kinase 1 and reduces its cofilin-phosphorylation activity via ubiquitination. Experimental Cell Research, 313(13), 2858–2874. doi:10.1016/j.yexcr.2007.04.016.
Bradke, F., & Dotti, C. G. (1999). The role of local actin instability in axon formation. Science, 283(5409), 1931–1934. doi:10.1126/science.283.5409.1931.
Geraldo, S., & Gordon-Weeks, P. R. (2009). Cytoskeletal dynamics in growth-cone steering. Journal of Cell Science, 122(20), 3595–3604. doi:10.1242/jcs.042309.
Parisiadou, L., Xie, C., Cho, H. J., Lin, X., Gu, X. L., Long, C. X., et al. (2009). Phosphorylation of ezrin/radixin/moesin proteins by LRRK2 promotes the rearrangement of actin cytoskeleton in neuronal morphogenesis. Journal of Neuroscience, 29(44), 13971–13980. doi:10.1523/JNEUROSCI.3799-09.2009.
Fierro-González, J. C., White, M. D., Silva, J. C., & Plachta, N. (2013). Cadherin-dependent filopodia control preimplantation embryo compaction. Nature Cell Biology, 15(12), 1424–1433. doi:10.1038/ncb2875.
Narumiya, S., Ishizaki, T., & Ufhata, M. (2000). Use and properties of ROCK-specific inhibitor Y-27632. In W. E. Balch, J. D. Channing, & H. Alan (Eds.), Methods in Enzymology (pp. 273–284). San Diego: Academic Press. doi:10.1016/S0076-6879(00)25449-9.
Acknowledgments
This work was supported by the PON project 254/Ric. “Implementation of human and environment health research center”, Cod. PONa3_00334, REA research Grant no PITN-GA-2012-316549 (IT LIVER) from the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007-2013), PRIN 2010FPTBSH “NANO Molecular technologies for Drug delivery - NANOMED”, Cod. PON02_00563_3448479-F “RINOVATIS”, and Cod. PONa3_00134 “Omics and nanotechnology for early diagnosis of human diseases - ONEV”. LLdM thanks Prof. Arezki Boudaoud for useful discussions about FibrilTool.
Author information
Authors and Affiliations
Corresponding author
Additional information
Daniele Vergara, Marzia M. Ferraro and Mariafrancesca Cascione have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Vergara, D., Ferraro, M.M., Cascione, M. et al. Cytoskeletal Alterations and Biomechanical Properties of parkin-Mutant Human Primary Fibroblasts. Cell Biochem Biophys 71, 1395–1404 (2015). https://doi.org/10.1007/s12013-014-0362-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0362-1