Abstract
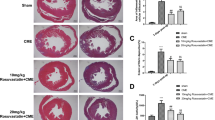
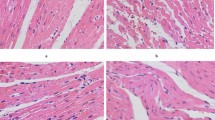
To observe the effect of simvastatin in patients with acute myocardial infarction in rabbits against myocardial apoptosis, and to explore its possible mechanism. Male New Zealand white rabbits were randomized into three groups, including the myocardial infarction group (12 rabbits), the simvastatin treatment group (15 rabbits), and the sham group (12 rabbits). In the simvastatin treatment and myocardial infarction groups, the rabbits received myocardial infarction surgeries. While in the sham group, loose knots were tied in the left anterior descending coronary artery branches. The simvastatin treatment group was given simvastatin by oral gavage 24 h after surgery. Parameters, which included left ventricular end-diastolic diameter, left ventricular end-systolic diameter, left ventricular ejection fraction, and left ventricular mass index, were recorded in these three groups. Edge myocardial infarction and myocardial cell apoptosis were analyzed using TUNEL assay, and Bcl-2, Bax, and Caspase-3 protein levels were detected by Western blot. Acute myocardial infarction model was successfully established in rabbits by ligation of the left anterior descending coronary artery. Compared with the myocardial infarction group, left ventricular end-diastolic diameter (LVEDD) and left ventricular end systolic diameter (LVESD) were significantly reduced and left ventricular ejection fraction (LVEF) increased in the simvastatin treatment group. Compared with the sham group, LVEDD and LVESD were significantly increased and LVEF decreased in the simvastatin treatment group. All the differences were statistically significant (P < 0.05). Left ventricular mass index in the simvastatin treatment group was statistically lower than the myocardial infarction group. Compared with the sham group, left ventricular mass index in both the simvastatin treatment and myocardial infarction groups was significantly increased. The differences of the above comparisons were statistically significant (P < 0.05). Compared with the sham group, the apoptosis rate of the myocardial infarction group and the simvastatin treatment groups was significantly increased as shown by TUNEL assay, however, the apoptosis rate of the simvastatin treatment group was significantly lower than that of the myocardial infarction group. All the differences among above comparisons were statistically significant (P < 0.05). Bcl-2 levels significantly increased in the simvastatin treatment group compared with the myocardial infarction group, but Bcl-2 levels in both groups were significantly lower than the sham group. However, Bax protein levels showed inverse expression with Bcl-2. Meanwhile, Caspase-3 protein expression showed similar trend with Bcl-2. Simvastatin can improve cardiac function after myocardial infarction and reduce apoptosis of myocardial cells, possibly by decreasing Bax and Caspase-3 expression and increasing the expression level of Bcl-2.




Similar content being viewed by others
References
Ibrahim, A. W., Riddell, T. C., & Devireddy, C. M. (2014). Acute myocardial infarction. Critical Care Clinics, 30, 341–364.
Nakatsuma, K., Shiomi, H., Watanabe, H., Morimoto, T., Taniguchi, T., Toyota, T., et al. (2014). Comparison of long-term mortality after acute myocardial infarction treated by percutaneous coronary intervention in patients living alone versus not living alone at the time of hospitalization. The American Journal of Cardiology, 114(4), 522–527.
Shiraishi, J., Kohno, Y., Nakamura, T., Yanagiuchi, T., Hashimoto, S., Ito, D., et al. (2014). Clinical impact of thrombus aspiration during primary percutaneous coronary intervention in acute myocardial infarction with occluded culprit. Cardiovascular Intervention and Therapeutics,. doi:10.1007/s12928-014-0282-4.
Kim, J., Ghasemzadeh, N., Eapen, D. J., Chung, N. C., Storey, J. D., Quyyumi, A. A., et al. (2014). Gene expression profiles associated with acute myocardial infarction and risk of cardiovascular death. Genome medicine, 6, 40.
Xiao, T. T., Wang, Y. Y., Zhang, Y., Bai, C. H., & Shen, X. C. (2014). Similar to spironolactone, oxymatrine is protective in aldosterone-induced cardiomyocyte injury via inhibition of calpain and apoptosis-inducing factor signaling. PLoS One, 9, e88856.
Yang, C., Liu, Z., Liu, K., & Yang, P. (2014). Mechanisms of Ghrelin anti-heart failure: Inhibition of Ang II-induced cardiomyocyte apoptosis by down-regulating AT1R expression. PLoS One, 9, e85785.
Lu, J., Sun, B., Huo, R., Wang, Y. C., Yang, D., Xing, Y., et al. (2014). Bone morphogenetic protein-2 antagonizes bone morphogenetic protein-4 induced cardiomyocyte hypertrophy and apoptosis. Journal of Cellular Physiology, 229, 1503–1510.
Nam, S. W., Liu, H., Wong, J. Z., Feng, A. Y., Chu, G., Merchant, N., et al. (2014). Cardiomyocyte apoptosis contributes to pathogenesis of cirrhotic cardiomyopathy in bile duct-ligated mice. Clinical Science (Lond), 127, 519–526.
Pastuszczak, M., Kotlarz, A., Mostowik, M., Zalewski, J., Zmudka, K., & Undas, A. (2010). Prior simvastatin treatment is associated with reduced thrombin generation and platelet activation in patients with acute ST-segment elevation myocardial infarction. Thrombosis Research, 125, 382–386.
Chenot, F., Montant, P. F., Marcovitch, O., Blaimont, M., de Meester, A., & Descamps, O. S. (2007). Co-administration of ezetimibe and simvastatin in acute myocardial infarction. European Journal of Clinical Investigation, 37, 357–363.
Waza, A. A., Andrabi, K., & Hussain, M. U. (2014). Protein kinase C (PKC) mediated interaction between conexin43 (Cx43) and K(ATP) channel subunit (Kir6.1) in cardiomyocyte mitochondria: Implications in cytoprotection against hypoxia induced cell apoptosis. Cellular Signalling, 26, 1909–1917.
Shin, J. H., Lim, Y. H., Song, Y. S., So, B. I., Park, J. Y., Fang, C. H., et al. (2014). Granulocyte-colony stimulating factor reduces cardiomyocyte apoptosis and ameliorates diastolic dysfunction in Otsuka long-evans Tokushima Fatty rats. Cardiovascular drugs and therapy/sponsored by the International Society of Cardiovascular Pharmacotherapy, 28, 211–220.
Sipkens, J. A., Krijnen, P. A., Hahn, N. E., Wassink, M., Meischl, C., Smith, D. E., et al. (2011). Homocysteine-induced cardiomyocyte apoptosis and plasma membrane flip-flop are independent of S-adenosylhomocysteine: A crucial role for nuclear p47(phox). Molecular and Cellular Biochemistry, 358, 229–239.
Xu, X. H., Xu, J., Xue, L., Cao, H. L., Liu, X., & Chen, Y. J. (2011). VEGF attenuates development from cardiac hypertrophy to heart failure after aortic stenosis through mitochondrial mediated apoptosis and cardiomyocyte proliferation. Journal of Cardiothoracic Surgery, 6, 54.
Boyle, A. J., Shih, H., Hwang, J., Ye, J., Lee, B., Zhang, Y., et al. (2011). Cardiomyopathy of aging in the mammalian heart is characterized by myocardial hypertrophy, fibrosis and a predisposition towards cardiomyocyte apoptosis and autophagy. Experimental Gerontology, 46, 549–559.
Ponce, N. E., Cano, R. C., Carrera-Silva, E. A., Lima, A. P., Gea, S., & Aoki, M. P. (2012). Toll-like receptor-2 and interleukin-6 mediate cardiomyocyte protection from apoptosis during Trypanosoma cruzi murine infection. Medical Microbiology and Immunology, 201, 145–155.
Verbrugge, F. H., Dupont, M., Rivero-Ayerza, M., de Vusser, P., Van Herendael, H., Vercammen, J., et al. (2012). Comorbidity significantly affects clinical outcome after cardiac resynchronization therapy regardless of ventricular remodeling. Journal of Cardiac Failure, 18, 845–853.
Choi, J. O., Kim, E. Y., Lee, G. Y., Lee, S. C., Park, S. W., Kim, D. K., et al. (2013). Predictors of left ventricular reverse remodeling and subsequent outcome in nonischemic dilated cardiomyopathy. Circulation Journal: Official Journal of the Japanese Circulation Society, 77, 462–469.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Luo, Kq., Long, Hb. & Xu, Bc. Reduced Apoptosis After Acute Myocardial Infarction by Simvastatin. Cell Biochem Biophys 71, 735–740 (2015). https://doi.org/10.1007/s12013-014-0257-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0257-1