Abstract
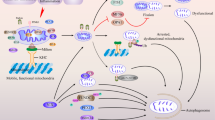
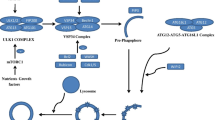
The autophagic process is the only known mechanism for mitochondrial turnover and it has been speculated that dysfunction of autophagy may result in mitochondrial error and cellular stress. Emerging investigations have provided new understanding of how autophagy of mitochondria (also known as mitophagy) is associated with cellular oxidative stress and its impact on neurodegeneration. This impaired autophagic function may be considered as a possible mechanism in the pathogenesis of several neurodegenerative disorders including Parkinson’s disease, Alzheimer’s disease, multiple sclerosis, amyotrophic lateral sclerosis, and Huntington disease. It can be suggested that autophagy dysfunction along with oxidative stress is considered main events in neurodegenerative disorders. New therapeutic approaches have now begun to target mitochondria as a potential drug target. This review discusses evidence supporting the notion that oxidative stress and autophagy are intimately associated with neurodegenerative disease pathogenesis. This review also explores new approaches that can prevent mitochondrial dysfunction, improve neurodegenerative etiology, and also offer possible cures to the aforementioned neurodegenerative diseases.


Similar content being viewed by others
Abbreviations
- AD:
-
Alzhiemers disease
- PD:
-
Parkinson disease
- ALS:
-
Amyotrophic lateral sclerosis
- HD:
-
Huntington’s disease
- ROS:
-
Reactive oxygen species
- MS:
-
Multiple sclerosis
References
Shacka, J. J., Roth, K. A., & Zhang, J. (2008). The autophagy–lysosomal degradation pathway: Role in neurodegenerative disease and therapy. Frontiers in Bioscience, 13, 718–736.
Cherra, S. J, 3rd, & Chu, C. T. (2008). Autophagy in neuroprotection and neurodegeneration: A question of balance. Future Neurology, 3, 309–323.
Chinnery, P. F., & Schon, E. A. (2003). Mitochondria. Journal of Neurology, Neurosurgery and Psychiatry, 74, 1188–1199.
Jellinger, K. A. (2009). Recent advances in our understanding of neurodegeneration. Journal of Neural Transmission, 9, 1111–1162.
Hashimoto, M., Rockenstein, E., Crews, L., & Masliah, E. (2003). Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. NeuroMolecular Medicine, 4, 21–36.
Cardaioli, E., Sicurelli, F., Carluccio, M. A., Gallus, G. N., Da Pozzo, P., Mondelli, M., et al. (2012). A new thymidine phosphorylase mutation causing elongation of the protein underlies mitochondrial neurogastrointestinal encephalomyopathy. Journal of Neurology, 259, 172–174.
Kamat, P. K., Tota, S., Shukla, R., Ali, S., Najmi, A. K., & Nath, C. (2011). Mitochondrial dysfunction: a crucial event in okadaic acid (ICV) induced memory impairment and apoptotic cell death in rat brain. Pharmacology, Biochemistry and Behavior, 100, 311–319.
Gavin, P. D., Prescott, M., & Devenish, R. J. (2005). F1F0-ATP synthase complex interactions in vivo can occur in the absence of the dimer specific subunit. Journal of Bioenergetics and Biomembranes, 37, 55–66.
Kim, I., Rodríguez-Enríquez, S., & Lemasters, J. J. (2007). Selective degradation of mitochondria by mitophagy. Archives of Biochemistry and Biophysics, 462, 245–253.
Jing, K., & Lim, K. (2012). Why is autophagy important in human diseases? Experimental & Molecular Medicine, 44, 69–72.
Gottlieb, R. A., & Carreira, R. S. (2010). Autophagy in health and disease. American Journal of Physiology - Cell Physiology, 299, C203–C210.
Komatsu, M., Waguri, S., Chiba, T., Murata, S., Iwata, J. I., Tanida, I., et al. (2006). Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature, 441, 880–884.
Banerjee, R., Beal, M. F., & Thomas, B. (2010). Autophagy in neurodegenerative disorders: Pathogenic roles and therapeutic implications. Trends in Neuroscience, 33, 541–549.
Wong, E., & Cuervo, A. M. (2010). Autophagy gone awry in neurodegenerative diseases. Nature Neuroscience, 13, 805–811.
Moreira, P. I., Santos, R. X., Zhu, X., Lee, H. G., Smith, M. A., Casadesus, G., et al. (2010). Autophagy in Alzheimer’s disease. Expert Review of Neurotherapeutics, 10, 1209–1218.
Yang, Y. P., Liang, Z. Q., Gu, Z. L., & Qin, Z. H. (2005). Molecular mechanism and regulation of autophagy. Acta Pharmacologica Sinica, 26, 1421–1434.
Ravikumar, B., Stewart, A., Kita, H., Kato, K., Duden, R., & Rubinsztein, D. C. (2003). Raised intracellular glucose concentrations reduce aggregation and cell death caused by mutant huntingtin exon 1 by decreasing mTOR phosphorylation and inducing autophagy. Human Molecular Genetics, 12, 985–994.
Webb, J. L., Ravikumar, B., Atkins, J., Skepper, J. N., & Rubinsztein, D. C. (2003). Alpha-Synuclein is degraded by both autophagy and the proteasome. Journal of Biological Chemistry, 278, 25009–25013.
Liu, B., Cheng, Y., Zhang, B., Bian, H. J., & Bao, J. K. (2009). Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria-mediated ROS-p38-p53 pathway. Cancer Letters, 275, 54–60.
Chu, C. T., Plowey, E. D., Dagda, R. K., Hickey, R. W., Cherra, S. J, 3rd, & Clark, R. S. (2009). Autophagy in neurite injury and neurodegeneration: In vitro and in vivo models. Methods in Enzymology, 453, 217–249.
Bossy, B., Perkins, G., & Bossy-Wetzel, E. (2008). Clearing the brain’s cobwebs: The role of autophagy in neuroprotection. Current Neuropharmacology, 6, 97–101.
Filosto, M., Scarpelli, M., Cotelli, M. S., Vielmi, V., Todeschini, A., Gregorelli, V., et al. (2011). The role of mitochondria in neurodegenerative diseases. Journal of Neurology, 258, 1763–1774.
Khan, M. U., Cheema, Y., Shahbaz, A. U., Ahokas, R. A., Sun, Y., Gerling, I. C., et al. (2012). Mitochondria play a central role in nonischemic cardiomyocyte necrosis: Common to acute and chronic stressor states. Pflugers Archiv, 464, 123–131.
Zheng, Y. Z., Berg, K. B., & Foster, L. J. (2009). Mitochondria do not contain lipid rafts, and lipid rafts do not contain mitochondrial proteins. Journal of Lipid Research, 50, 988–998.
Santos, J. M., & Kowluru, R. A. (2011). Role of mitochondria biogenesis in the metabolic memory associated with the continued progression of diabetic retinopathy and its regulation by lipoic acid. Investigative Ophthalmology & Visual Science, 52, 8791–8798.
Parsons, M. J., & Green, D. R. (2009). Mitochondria and apoptosis: a quick take on a long view. F1000 Biology Reports, 1, 17.
Palikaras, K., & Tavernarakis, N. (2012). Mitophagy in neurodegeneration and aging. Frontiers in Genetics, 3, 297.
Hagen, T., Lagace, C. J., Modica-Napolitano, J. S., & Aprille, J. R. (2003). Permeability transition in rat liver mitochondria is modulated by the ATP-Mg/Pi carrier. American Journal of Physiology: Gastrointestinal and Liver Physiology, 285, G274–G281.
Wallace, D. C. (2005). Mitochondria and cancer: Warburg addressed. Cold Spring Harbor Symposia on Quantitative Biology, 70, 363–374.
Karbowski, M. (2010). Mitochondria on guard: Role of mitochondrial fusion and fission in the regulation of apoptosis. Advances in Experimental Medicine and Biology, 687, 131–142.
Langer, T., Kaser, M., Klanner, C., & Leonhard, K. (2001). AAA proteases of mitochondria: Quality control of membrane proteins and regulatory functions during mitochondrial biogenesis. Biochemical Society Transactions, 29, 431–436.
Soubannier, V., McLelland, G. L., Zunino, R., Braschi, E., Rippstein, P., Fon, E. A., et al. (2012). A vesicular transport pathway shuttles cargo from mitochondria to lysosomes. Current Biology, 22, 135–141.
Miwa, S., St-Pierre, J., Partridge, L., & Brand, M. D. (2003). Superoxide and hydrogen peroxide production by Drosophila mitochondria. Free Radical Biology & Medicine, 35, 938–948.
Twig, G., Graf, S. A., Wikstrom, J. D., Mohamed, H., Haigh, S. E., Elorza, A., et al. (2006). Tagging and tracking individual networks within a complex mitochondrial web with photoactivatable GFP. American Journal of Physiology Cell Physiology, 291, C176–C184.
Twig, G., Elorza, A., Molina, A. J., Mohamed, H., Wikstrom, J. D., Walzer, G., et al. (2008). Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO Journal, 27, 433–446.
Frieden, M., Arnaudeau, S., Castelbou, C., & Demaurex, N. (2005). Subplasmalemmal mitochondria modulate the activity of plasma membrane Ca2 + -ATPases. Journal of Biological Chemistry, 280, 43198–43208.
Gruber, J., Fong, S., Chen, C. B., Yoong, S., Pastorin, G., Schaffer, S., et al. (2012). Mitochondria-targeted antioxidants and metabolic modulators as pharmacological interventions to slow ageing. Biotechnology Advances. doi:10.1016/j.biotechadv.2012.09.005.
Chen, J., Tang, X. Q., Zhi, J. L., Cui, Y., Yu, H. M., Tang, E. H., et al. (2006). Curcumin protects PC12 cells against 1-methyl-4-phenylpyridinium ion-induced apoptosis by bcl-2-mitochondria-ROS-iNOS pathway. Apoptosis, 11, 943–953.
Tang, L., Zhang, Y., Jobson, H. E., Li, J., Stephenson, K. K., Wade, K. L., et al. (2006). Potent activation of mitochondria-mediated apoptosis and arrest in S and M phases of cancer cells by a broccoli sprout extract. Molecular Cancer Therapeutics, 5, 935–944.
Lemasters, J. J. (2005). Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Research, 8, 3–5.
Lin, M. T., & Beal, M. F. (2006). Alzheimer’s APP mangles mitochondria. Nature Medicine, 12, 1241–1243.
Frazziano, G., Champion, H. C., & Pagano, P. J. (2012). NADPH oxidase-derived ROS and the regulation of pulmonary vessel tone. American Journal of Physiology Heart and Circulatory Physiology, 302, H2166–H2177.
Takahashi, E., Endoh, H., & Doi, K. (1999). Intracellular gradients of O2 supply to mitochondria in actively respiring single cardiomyocyte of rats. American Journal of Physiology, 276, H718–H724.
McKenna, M. C. (2011). Glutamate dehydrogenase in brain mitochondria: Do lipid modifications and transient metabolon formation influence enzyme activity? Neurochemistry International, 59, 525–533.
Nordberg, J., & Arner, E. S. (2001). Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radical Biology & Medicine, 31, 1287–1312.
Turrens, J. F. (2003). Mitochondrial formation of reactive oxygen species. Journal of Physiology, 552, 335–344.
Lundberg, I. E. (2002). New possibilities to achieve increased understanding of disease mechanisms in idiopathic inflammatory myopathies. Current Opinion in Rheumatology, 14, 639–642.
Burdon, R. H., Gill, V., Boyd, P. A., & Rahim, R. A. (1996). Hydrogen peroxide and sequence-specific DNA damage in human cells. FEBS Letters, 383, 150–154.
Rubinsztein, D. C., DiFiglia, M., Heintz, N., Nixon, R. A., Qin, Z. H., Ravikumar, B., et al. (2005). Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy, 1, 11–22.
Galluzzi, L., Blomgren, K., & Kroemer, G. (2009). Mitochondrial membrane permeabilization in neuronal injury. Nature Reviews Neuroscience, 10, 481–494.
Pinton, P., Giorgi, C., Siviero, R., Zecchini, E., & Rizzuto, R. (2008). Calcium and apoptosis: ER-mitochondria Ca2 + transfer in the control of apoptosis. Oncogene, 27, 6407–6418.
Zecchini, V., & Mills, I. G. (2009). Putting chromatin immunoprecipitation into context. Journal of Cellular Biochemistry, 107, 19–29.
Rogawski, M. A., & Wenk, G. L. (2003). The neuropharmacological basis for the use of memantine in the treatment of Alzheimer’s disease. CNS Drug Reviews, 9, 275–308.
Cardoso, S. M., Santana, I., Swerdlow, R. H., & Oliveira, C. R. (2004). Mitochondria dysfunction of Alzheimer’s disease cybrids enhances Abeta toxicity. Journal of Neurochemistry, 89, 1417–1426.
Butterfield, D. A., & Lauderback, C. M. (2002). Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radical Biology & Medicine, 32, 1050–1060.
Aksenov, M. Y., Aksenova, M. V., Butterfield, D. A., Geddes, J. W., & Markesbery, W. R. (2000). Protein oxidation in the brain in Alzheimer’s disease. Neuroscience, 103, 373–383.
Maruszak, A., & Zekanowski, C. (2011). Mitochondrial dysfunction and Alzheimer’s disease. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 35, 320–330.
Butler, D., Nixon, R. A., & Bahr, B. A. (2006). Potential compensatory responses through autophagic/lysosomal pathways in neurodegenerative diseases. Autophagy, 2, 234–237.
Nixon, R. A., Wegiel, J., Kumar, A., Yu, W. H., Peterhoff, C., Cataldo, A., et al. (2005). Extensive involvement of autophagy in Alzheimer disease: an immuno-electron microscopy study. Neuropathology & Experimental Neurology, 64, 113–122.
Nixon, R. A. (2007). Autophagy, amyloidogenesis and Alzheimer disease. Journal of Cell Science, 120, 4081–4091.
Kirik, D., Rosenblad, C., Burger, C., Lundberg, C., Johansen, T. E., Muzyczka, N., et al. (2002). Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. Journal of Neuroscience, 22, 2780–2791.
Tian, L., Ma, L., Kaarela, T., & Li, Z. (2012). Neuroimmune crosstalk in the central nervous system and its significance for neurological diseases. Journal of Neuroinflammation, 9, 155.
Battisti, C., Formichi, P., Radi, E., & Federico, A. (2008). Oxidative-stress-induced apoptosis in PBLs of two patients with Parkinson disease secondary to alpha-synuclein mutation. Journal of the Neurological Sciences, 267, 120–124.
Alvarez-Erviti, L., Rodriguez-Oroz, M. C., Cooper, J. M., Caballero, C., Ferrer, I., Obeso, J. A., et al. (2010). Chaperone-mediated autophagy markers in Parkinson disease brains. Archives of Neurology, 67, 1464–1472.
Rogaeva, E., Johnson, J., Lang, A. E., Gulick, C., Gwinn-Hardy, K., Kawarai, T., et al. (2004). Analysis of the PINK1 gene in a large cohort of cases with Parkinson disease. Archives of Neurology, 61, 1898–1904.
Yang, L., Xia, Y., Zhao, H., Zhao, J., & Zhu, X. (2006). Magnetic resonance imaging of transplanted neural stem cells in Parkinson disease rats. Journal of Huazhong University of Science and Technology (Medical Sciences), 26, 489–492.
Banerjee, R., Starkov, A. A., Beal, M. F., & Thomas, B. (2009). Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochimica et Biophysica Acta, 7, 651–663.
Narendra, D., Tanaka, A., Suen, D. F., & Youle, R. J. (2008). Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. Journal of Cell Biology, 183, 795–803.
Chu, C. T., Zhu, J., & Dagda, R. (2007). Beclin 1-independent pathway of damage-induced mitophagy and autophagic stress: Implications for neurodegeneration and cell death. Autophagy, 3, 663–666.
Dagda, R. K., Cherra, S. J, 3rd, Kulich, S. M., Tandon, A., Park, D., & Chu, C. T. (2009). Loss of PINK1 function promotes mitophagy through effects on oxidative stress and mitochondrial fission. Journal of Biological Chemistry, 284, 13843–13855.
McBride, H. M. (2008). Parkin mitochondria in the autophagosome. Journal of Cell Biology, 183, 757–759.
Greenamyre, J. T., Sherer, T. B., Betarbet, R., & Panov, A. V. (2001). Complex I and Parkinson’s disease. IUBMB Life, 52, 135–141.
Seet, R. C., Lee, C. Y., Lim, E. C., Tan, J. J., Quek, A. M., Chong, W. L., et al. (2010). Oxidative damage in Parkinson disease: Measurement using accurate biomarkers. Free Radical Biology & Medicine, 48, 560–566.
Bender, A., Krishnan, K. J., Morris, C. M., Taylor, G. A., Reeve, A. K., Perry, R. H., et al. (2006). High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nature Genetics, 38, 515–517.
Dalfo, E., & Ferrer, I. (2005). Alpha-synuclein binding to rab3a in multiple system atrophy. Neuroscience Letters, 380, 170–175.
Murphy, B. M., Dandy, D. S., & Henry, C. S. (2009). Analysis of oxidative stress biomarkers using a simultaneous competitive/non-competitive micromosaic immunoassay. Analytica Chimica Acta, 640, 1–6.
Chen, C. Y., Jang, J. H., Park, M. H., Hwang, S. J., Surh, Y. J., & Park, O. J. (2007). Attenuation of Abeta-induced apoptosis of plant extract (Saengshik) mediated by the inhibition of mitochondrial dysfunction and antioxidative effect. Annals of the New York Academy of Sciences, 1095, 399–411.
Dodson, M. W., & Guo, M. (2007). Pink1, Parkin, DJ-1 and mitochondrial dysfunction in Parkinson’s disease. Current Opinion in Neurobiology, 17, 331–337.
Pavese, N., Gerhard, A., Tai, Y. F., Ho, A. K., Turkheimer, F., Barker, R. A., et al. (2006). Microglial activation correlates with severity in Huntington disease: A clinical and PET study. Neurology, 66, 1638–1643.
Gusella, J. F., & MacDonald, M. E. (1995). Huntington’s disease: CAG genetics expands neurobiology. Current Opinion in Neurobiology, 5, 656–662.
Kegel, K. B., Kim, M., Sapp, E., McIntyre, C., Castano, J. G., Aronin, N., et al. (2000). Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy. Journal of Neuroscience, 20, 7268–7278.
Orr, A. L., Li, S., Wang, C. E., Li, H., Wang, J., Rong, J., et al. (2008). N-terminal mutant huntingtin associates with mitochondria and impairs mitochondrial trafficking. Journal of Neuroscience, 28, 2783–2792.
Panov, A. V., Gutekunst, C. A., Leavitt, B. R., Hayden, M. R., Burke, J. R., Strittmatter, W. J., et al. (2002). Early mitochondrial calcium defects in Huntington’s disease are a direct effect of polyglutamines. Nature Neuroscience, 5, 731–736.
Modugno, N., Curra, A., Giovannelli, M., Priori, A., Squitieri, F., Ruggieri, S., et al. (2001). The prolonged cortical silent period in patients with Huntington’s disease. Clinical Neurophysiology, 112, 1470–1474.
Herishanu, Y. O., Parvari, R., Pollack, Y., Shelef, I., Marom, B., Martino, T., et al. (2009). Huntington disease in subjects from an Israeli Karaite community carrying alleles of intermediate and expanded CAG repeats in the HTT gene: Huntington disease or phenocopy? Journal of the Neurological Sciences, 277, 143–146.
Tellez-Nagel, I., Johnson, A. B., & Terry, R. D. (1974). Studies on brain biopsies of patients with Huntington’s chorea. Journal of Neuropathology and Experimental Neurology, 33, 308–332.
Hirano, M. (2008). VAPB: new genetic clues to the pathogenesis of ALS. Neurology, 70, 1161–1162.
Martinez-Vicente, M., Talloczy, Z., Wong, E., Tang, G., Koga, H., Kaushik, S., et al. (2010). Cargo recognition failure is responsible for inefficient autophagy in Huntington’s disease. Nature Neuroscience, 13, 567–576.
Kawamata, H., & Manfredi, G. (2010). Mitochondrial dysfunction and intracellular calcium dysregulation in ALS. Mechanisms of Ageing and Development, 131, 517–526.
Julien, J. P. (2007). ALS: Astrocytes move in as deadly neighbors. Nature Neuroscience, 10, 535–537.
Liu, B., Tewari, A. K., Zhang, L., Green-Church, K. B., Zweier, J. L., Chen, Y. R., et al. (2009). Proteomic analysis of protein tyrosine nitration after ischemia reperfusion injury: Mitochondria as the major target. Biochimica et Biophysica Acta, 1794, 476–485.
Beckman, J. S., Estevez, A. G., Crow, J. P., & Barbeito, L. (2001). Superoxide dismutase and the death of motoneurons in ALS. Trends in Neurosciences, 24, S15–S20.
Okamoto, K., Fujita, Y., & Mizuno, Y. (2010). Pathology of protein synthesis and degradation systems in ALS. Neuropathology, 30, 189–193.
Li, B., Liu, X. Y., Li, Z., Bu, H., Sun, M. M., Guo, Y. S., et al. (2008). Effect of ALS IgG on motor neurons in organotypic spinal cord cultures. Canadian Journal of Neurological Sciences, 35, 220–225.
Shi, P., Strom, A. L., Gal, J., & Zhu, H. (2010). Effects of ALS-related SOD1 mutants on dynein- and KIF5-mediated retrograde and anterograde axonal transport. Biochimica et Biophysica Acta, 1802, 707–716.
Rosen, D. R., Siddique, T., Patterson, D., Figlewicz, D. A., Sapp, P., Hentati, A., et al. (1993). Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature, 362, 59–62.
Roxburgh, R. H., Seaman, S. R., Masterman, T., Hensiek, A. E., Sawcer, S. J., Vukusic, S., et al. (2005). Multiple Sclerosis Severity Score: Using disability and disease duration to rate disease severity. Neurology., 64, 1144–1151.
Noseworthy, J., Kappos, L., & Daumer, M. (2003). Competing interests in multiple sclerosis research. Lancet, 361, 350–351.
Waxman, S. G. (2008). Axonal dysfunction in chronic multiple sclerosis: Meltdown in the membrane. Annals of Neurology, 63, 411–413.
Mahad, D., Ziabreva, I., Lassmann, H., & Turnbull, D. (2008). Mitochondrial defects in acute multiple sclerosis lesions. Brain, 131, 1722–1735.
Young, E. A., Fowler, C. D., Kidd, G. J., Chang, A., Rudick, R., Fisher, E., et al. (2008). Imaging correlates of decreased axonal Na +/K + ATPase in chronic multiple sclerosis lesions. Annals of Neurology, 63, 428–435.
Neymotin, A., Calingasan, N. Y., Wille, E., Naseri, N., Petri, S., Damiano, M., et al. (2011). Neuroprotective effect of Nrf2/ARE activators, CDDO ethylamide and CDDO trifluoroethylamide, in a mouse model of amyotrophic lateral sclerosis. Free Radical Biology & Medicine, 51, 88–96.
Stack, C., Ho, D., Wille, E., Calingasan, N. Y., Williams, C., Liby, K., et al. (2010). Triterpenoids CDDO-ethyl amide and CDDO-trifluoroethyl amide improve the behavioral phenotype and brain pathology in a transgenic mouse model of Huntington’s disease. Free Radical Biology and Medicine, 49, 147–158.
Sauerbeck, A., Gao, J., Readnower, R., Liu, M., Pauly, J. R., Bing, G., et al. (2011). Pioglitazone attenuates mitochondrial dysfunction, cognitive impairment, cortical tissue loss, and inflammation following traumatic brain injury. Experimental Neurology, 227, 128–135.
Ghosh, S., Patel, N., Rahn, D., McAllister, J., Sadeghi, S., Horwitz, G., et al. (2007). The thiazolidinedione pioglitazone alters mitochondrial function in human neuron-like cells. Molecular Pharmacology, 71, 1695–1702.
Quintanilla, R. A., Jin, Y. N., Fuenzalida, K., Bronfman, M., & Johnson, G. V. (2008). Rosiglitazone treatment prevents mitochondrial dysfunction in mutant huntingtin-expressing cells: possible role of peroxisome proliferator-activated receptor-gamma (PPARgamma) in the pathogenesis of Huntington disease. Journal of Biological Chemistry, 283, 25628–25637.
Albert, Y. Sun. (2010). Resveratrol as a therapeutic agent for neurodegenerative diseases. Molecular Neurobiology, 41, 375–383.
Ayasolla, K. R., Giri, S., Singh, A. K., & Singh, I. (2005). 5-aminoimidazole-4-carboxamide-1-beta-4-ribofuranoside (AICAR) attenuates the expression of LPS- and Aβ peptide-induced inflammatory mediators in astroglia. Journal of Neuroinflammation, 2, 21.
Orsucci, D., Mancuso, M., Ienco, E. C., LoGerfo, A., & Siciliano, G. (2011). Targeting mitochondrial dysfunction and neurodegeneration by means of coenzyme Q10 and its analogues. Current Medicinal Chemistry, 18, 4053–4064.
Michelangelo, Mancuso, Daniele, Orsucci, Valeria, Calsolaro, Anna, Choub, & Gabriele, Siciliano. (2009). Coenzyme Q10 and neurological diseases. Pharmaceuticals, 2, 134–149.
Noe, N., Dillon, L., Lellek, V., Diaz, F., Hida, A., Moraes, C. T., et al. (2012). Bezafibrate improves mitochondrial function in the CNS of a mouse model of mitochondrial encephalopathy. Mitochondrion, 12, 258–259.
Johri, A., Calingasan, N. Y., Hennessey, T. M., Sharma, A., Yang, L., Wille, E., et al. (2012). Pharmacologic activation of mitochondrial biogenesis exerts widespread beneficial effects in a transgenic mouse model of Huntington’s disease. Human Molecular Genetics, 21, 1124–1137.
Acknowledgments
This work was supported by the National Institutes of Health grants HL107640-NT.
Conflict of interest
Authors having no conflict of interest
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kamat, P.K., Kalani, A., Kyles, P. et al. Autophagy of Mitochondria: A Promising Therapeutic Target for Neurodegenerative Disease. Cell Biochem Biophys 70, 707–719 (2014). https://doi.org/10.1007/s12013-014-0006-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-014-0006-5