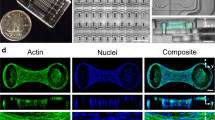
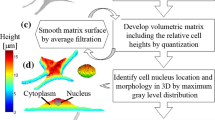
Abstract
The ability to harvest and maintain viable cells from mammalian tissues represented a critical advance in biomedical research, enabling individual cells to be cultured and studied in molecular detail. However, in these traditional cultures, cells are grown on rigid glass or polystyrene substrates, the mechanical properties of which often do not match those of the in vivo tissue from which the cells were originally derived. This mechanical mismatch likely contributes to abrupt changes in cellular phenotype. In fact, it has been proposed that mechanical changes in the cellular microenvironment may alone be responsible for driving specific cellular behaviors. Recent multidisciplinary efforts from basic scientists and engineers have begun to address this hypothesis more explicitly by probing the effects of ECM mechanics on cell and tissue function. Understanding the consequences of such mechanical changes is physiologically relevant in the context of a number of tissues in which altered mechanics may either correlate with or play an important role in the onset of pathology. Examples include changes in the compliance of blood vessels associated with atherosclerosis and intimal hyperplasia, as well as changes in the mechanical properties of developing tumors. Compelling evidence from 2-D in vitro model systems has shown that substrate mechanical properties induce changes in cell shape, migration, proliferation, and differentiation, but it remains to be seen whether or not these same effects translate to 3-D systems or in vivo. Furthermore, the molecular “mechanotransduction” mechanisms by which cells respond to changes in ECM mechanics remain unclear. Here, we provide some historical context for this emerging area of research, and discuss recent evidence that regulation of cytoskeletal tension by changes in ECM mechanics (either directly or indirectly) may provide a critical switch that controls cell function.
Similar content being viewed by others
References
Adams, J. C., & Watt, F. M. (1993). Regulation of development and differentiation by the extracellular matrix. Development, 117(4), 1183–1198.
Aikawa, M., Kim, H. S., Kuro-o, M., Manabe, I., Watanabe, M., Yamaguchi, H., Yazaki, Y., & Nagai, R. (1995). Phenotypic modulation of smooth muscle cells during progression of human atherosclerosis as determined by altered expression of myosin heavy chain isoforms. Annals of the New York Academy of Sciences, 748, 578–585.
Aikawa, R., Komuro, I., Yamazaki, T., Zou, Y., Kudoh, S., Zhu, W., Kadowaki, T., & Yazaki, Y. (1999). Rho family small G proteins play critical roles in mechanical stress-induced hypertrophic responses in cardiac myocytes. Circulation Research, 84(4), 458–466.
Aikawa, M., Sakomura, Y., Ueda, M., Kimura, K., Manabe, I., Ishiwata, S., Komiyama, N., Yamaguchi, H., Yazaki, Y., & Nagai, R. (1997). Redifferentiation of smooth muscle cells after coronary angioplasty determined via myosin heavy chain expression. Circulation, 96(1), 82–90.
Alenghat, F. J., & Ingber, D. E. (2002). Mechanotransduction: All signals point to cytoskeleton, matrix, and integrins. Science STKE, 2002(119), PE6.
Almany, L., & Seliktar, D. (2005). Biosynthetic hydrogel scaffolds made from fibrinogen and polyethylene glycol for 3D cell cultures. Biomaterials, 26(15), 2467–2477.
Amano, M., Chihara, K., Kimura, K., Fukata, Y., Nakamura, N., Matsuura, Y., & Kaibuchi, K. (1997). Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science, 275(5304), 1308–1311.
Aoki, H., Izumo, S., & Sadoshima, J. (1998). Angiotensin II activates RhoA in cardiac myocytes: A critical role of RhoA in angiotensin II-induced premyofibril formation. Circulation Research, 82(6), 666–676.
Arthur, W. T., & Burridge, K. (2001). RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Molecular Biology of the Cell, 12(9), 2711–2720.
Arthur, W. T., Petch, L. A., & Burridge, K. (2000). Integrin engagement suppresses RhoA activity via a c-Src-dependent mechanism. Current Biology, 10(12), 719–722.
Balaban, N. Q., Schwarz, U. S., Riveline, D., Goichberg, P., Tzur, G., Sabanay, I., Mahalu, D., Safran, S., Bershadsky, A., Addadi, L., & Geiger, B. (2001). Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nature Cell Biology, 3(5), 466–472.
Banes, A. J., Tsuzaki, M., Yamamoto, J., Fischer, T., Brigman, B., Brown, T., & Miller, L. (1995). Mechanoreception at the cellular level: The detection, interpretation, and diversity of responses to mechanical signals. Biochemistry and Cell Biology, 73(7–8), 349–365.
Barry, S. T., Flinn, H. M., Humphries, M. J., Critchley, D. R., & Ridley, A. J. (1997). Requirement for Rho in integrin signalling. Cell Adhesion and Communication, 4(6), 387–398.
Beningo, K. A., Dembo, M., Kaverina, I., Small, J. V., & Wang, Y. L. (2001). Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. Journal of Cell Biology, 153(4), 881–888.
Bernard, M. P., Myers, J. C., Chu, M. L., Ramirez, F., Eikenberry, E. F., & Prockop, D. J. (1983). Structure of a cDNA for the pro alpha 2 chain of human type I procollagen. Comparison with chick cDNA for pro alpha 2(I). identifies structurally conserved features of the protein and the gene. Biochemistry, 22(5), 1139–1145.
Bershadsky, A. D., Balaban, N. Q., & Geiger, B. (2003). Adhesion-dependent cell mechanosensitivity. Annual Review of Cell and Developmental Biology, 19, 677–695.
Bischofs, I. B., & Schwarz, U. S. (2003). Cell organization in soft media due to active mechanosensing. Proceedings of the National Academy of Sciences, 100(16), 9274–9279.
Brangwynne, C. P., Mackintosh, F. C., Kumar, S., Geisse, N. A., Talbot, J., Mahadevan, L., Parker, K. K., Ingber, D. E., & Weitz, D. A. (2006). Microtubules can bear enhanced compressive loads in living cells because of lateral reinforcement. Journal of Cell Biology, 173(5), 733–741.
Brown, E., McKee, T., diTomaso, E., Pluen, A., Seed, B., Boucher, Y., & Jain, R. K. (2003). Dynamic imaging of collagen and its modulation in tumors in␣vivo using second-harmonic generation. Nature Medicine, 9(6), 796–800.
Brown, X. Q., Ookawa, K., & Wong, J. Y. (2005). Evaluation of polydimethylsiloxane scaffolds with physiologically-relevant elastic moduli: Interplay of substrate mechanics and surface chemistry effects on vascular smooth muscle cell response. Biomaterials, 26(16), 3123–3129.
Bryant, S. J., & Anseth, K. S. (2002). Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. Journal of Biomedical Material Research, 59(1), 63–72.
Bryant, S. J., & Anseth, K. S. (2003). Controlling the spatial distribution of ECM components in degradable PEG hydrogels for tissue engineering cartilage. Journal of Biomedical Material Research A, 64(1), 70–79.
Bryant, S. J., Bender, R. J., Durand, K. L., & Anseth, K. S. (2004). Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: Engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnology and Bioengineering, 86(7), 747–755.
Burdick, J. A., & Anseth, K. S. (2002). Photoencapsulation of osteoblasts in injectable RGD-modified PEG hydrogels for bone tissue engineering. Biomaterials, 23(22), 4315–4323.
Burdick, J. A., Khademhosseini, A., & Langer, R. (2004). Fabrication of gradient hydrogels using a microfluidics/photopolymerization process. Langmuir, 20(13), 5153–5156.
Carmeliet, P. (2003). Angiogenesis in health and disease. Nature Medicine, 9(6), 653–660.
Chen, J., Fabry, B., Schiffrin, E. L., & Wang, N. (2001). Twisting integrin receptors increases endothelin-1 gene expression in endothelial cells. American Journal of Physiology Cell Physiology, 280(6), C1475–C1484.
Chen, K. D., Li, Y. S., Kim, M., Li, S., Yuan, S., Chien, S., & Shyy, J. Y. (1999). Mechanotransduction in response to shear stress. Roles of receptor tyrosine kinases, integrins, and Shc. Journal of Biological Chemistry, 274(26), 18393–18400.
Chen, C. S., Mrksich, M., Huang, S., Whitesides, G. M., & Ingber, D. E. (1997). Geometric control of cell life and death. Science, 276(5317), 1425–1428.
Chicurel, M. E., Chen, C. S., & Ingber, D. E. (1998). Cellular control lies in the balance of forces. Current Opinion in Cell Biology, 10(2), 232–239.
Chiquet, M. (1999). Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biology, 18(5), 417–426.
Chiquet, M., Matthisson, M., Koch, M., Tannheimer, M., & Chiquet-Ehrismann, R. (1996). Regulation of extracellular matrix synthesis by mechanical stress. Biochemistry and Cell Biology, 74(6), 737–744.
Chiquet, M., Renedo, A. S., Huber, F., & Fluck, M. (2003). How do fibroblasts translate mechanical signals into changes in extracellular matrix production? Matrix Biology, 22(1), 73–80.
Chong, L. D., Traynor-Kaplan, A., Bokoch, G. M., & Schwartz, M. A. (1994). The small GTP-binding protein Rho regulates a phosphatidylinositol 4- phosphate 5-kinase in mammalian cells. Cell, 79(3), 507–513.
Choquet, D., Felsenfeld, D. P., & Sheetz, M. P. (1997). Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell, 88(1), 39–48.
Chrzanowska-Wodnicka, M., Burridge, K. (1996). Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. Journal of Cell Biology, 133(6), 1403–1415.
Clark, E. A., King, W. G., Brugge, J. S., Symons, M., & Hynes, R. O. (1998). Integrin-mediated signals regulated by members of the rho family of GTPases. Journal of Cell Biology, 142(2), 573–586.
Cook, T. A., Nagasaki, T., & Gundersen, G. G. (1998). Rho guanosine triphosphatase mediates the selective stabilization of microtubules induced by lysophosphatidic acid. Journal of Cell Biology, 141(1), 175–185.
Cotran, R. S., Kumar, V., & Collins, T. (1999) Robbins pathologic basis of disease (6th ed.). Philadelphia: W.B. Saunders, p. 1425.
Cukierman, E., Pankov, R., Stevens, D. R., & Yamada, K. M. (2001). Taking cell-matrix adhesions to the third dimension. Science, 294(5547), 1708–1712.
Danowski, B. A. (1989). Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. Journal of Cell Science, 93(Pt 2), 255–266.
Darling, E. M., & Athanasiou, K. A. (2003). Articular cartilage bioreactors and bioprocesses. Tissue Engineering, 9(1), 9–26.
Davis, G. E., Bayless, K. J., & Mavila, A. (2002). Molecular basis of endothelial cell morphogenesis in three-dimensional extracellular matrices. Anatomical Record, 268(3), 252–275.
Dedhar, S., Ruoslahti, E., & Pierschbacher, M. D. (1987). A cell surface receptor complex for collagen type I recognizes the Arg-Gly-Asp sequence. Journal of Cell Biology, 104(3), 585–593.
Deroanne, C. F., Lapiere, C. M., & Nusgens, B. V. (2001). In vitro tubulogenesis of endothelial cells by relaxation of the coupling extracellular matrix-cytoskeleton. Cardiovascular Research, 49(3), 647–658.
Dikovsky, D., Bianco-Peled, H., & Seliktar, D. (2006). The effect of structural alterations of PEG-fibrinogen hydrogel scaffolds on 3-D cellular morphology and cellular migration. Biomaterials, 27(8), 1496–1506.
DiMilla, P. A., Stone, J. A., Quinn, J. A., Albelda, S. M., & Lauffenburger, D. A. (1993). Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. Journal of Cell Biology, 122(3), 729–737.
Discher, D. E., Janmey, P., & Wang, Y. L. (2005). Tissue cells feel and respond to the stiffness of their substrate. Science, 310(5751), 1139–1143.
Duncan, R. L. (1995). Transduction of mechanical strain in bone. ASGSB Bulletin, 8(2), 49–62.
Dvorak, H. F., Senger, D. R., & Dvorak, A. M. (1983). Fibrin as a component of the tumor stroma: Origins and biological significance. Cancer Metastasis Reviews, 2(1), 41–73.
Egeblad, M., & Werb, Z. (2002). New functions for the matrix metalloproteinases in cancer progression. Nature Reviews Cancer, 2(3), 161–174.
Elisseeff, J., Anseth, K., Sims, D., McIntosh, W., Randolph, M., & Langer, R. (1999). Transdermal photopolymerization for minimally invasive implantation. Proceedings of the National Academy of Sciences of the United States of America, 96(6), 3104–3107.
Engler, A., Bacakova, L., Newman, C., Hategan, A., Griffin, M., & Discher, D. (2004). Substrate compliance versus ligand density in cell on gel responses. Biophysical Journal, 86(1 Pt 1), 617–628.
Engler, A. J., Griffin, M. A., Sen, S., Bonnemann, C. G., Sweeney, H. L., & Discher, D. E. (2004). Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. Journal of Cell Biology, 166(6), 877–887.
Engler, A. J., Sen, S., Sweeney, H. L., & Discher, D. E. (2006). Matrix elasticity directs stem cell lineage specification. Cell, 126(4), 677–689.
Enomoto, T. (1996). Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: Possible involvement of the rho signal cascade. Cell Structure and Function, 21(5), 317–326.
Etienne-Manneville, S., & Hall, A. (2002). Rho GTPases in cell biology. Nature, 420(6916), 629–635.
Felsenfeld, D. P., Schwartzberg, P. L., Venegas, A., Tse, R., & Sheetz, M. P. (1999). Selective regulation of integrin–cytoskeleton interactions by the tyrosine kinase Src. Nature Cell Biology, 1(4), 200–206.
Folkman, J., & Moscona, A. (1978). Role of cell shape in growth control. Nature, 273(5661), 345–349.
Freyman, T. M., Yannas, I. V., Yokoo, R., & Gibson, L. J. (2002). Fibroblast contractile force is independent of the stiffness which resists the contraction. Experimental Cell Research, 272(2), 153–162.
Fringer, J., & Grinnell, F. (2001). Fibroblast quiescence in floating or released collagen matrices: Contribution of the ERK signaling pathway and actin cytoskeletal organization. Journal of Biological Chemistry, 276(33), 31047–31052.
Fritz, G., Just, I., & Kaina, B. (1999). Rho GTPases are over-expressed in human tumors. International Journal of Cancer, 81(5), 682–687.
Fuller, B. (1961). Tensegrity. Portfolio Artnews Annual, 4, 112–127.
Fung, Y. C. (1993). Biomechanics: Mechanical properties of living tissues (2nd ed.). New York: Springer-Verlag, p 568.
Galbraith, C. G., & Sheetz, M. P. (1998). Forces on adhesive contacts affect cell function. Current Opinion in Cell Biology, 10(5), 566–571.
Galbraith, C. G., Yamada, K. M., & Sheetz, M. P. (2002). The relationship between force and focal complex development. Journal of Cell Biology, 159(4), 695–705.
Genes, N. G., Rowley, J. A., Mooney, D. J., & Bonassar, L. J. (2004). Effect of substrate mechanics on chondrocyte adhesion to modified alginate surfaces. Archives of Biochemistry and Biophysics, 422(2), 161–167.
Georges, P. C., & Janmey, P. A. (2005). Cell type-specific response to growth on soft materials. Journal of Applied Physiology, 98(4), 1547–1553.
Ghajar, C. M., Blevins, K. S., Hughes, C. C. W., George, S. C., & Putnam, A. J. (2006). Mesenchymal stem cells enhance angiogenesis in mechanically viable prevascularized tissues via early MMP upregulation. Tissue Engineering, 12(10), 2875–2888.
Giancotti, F. G., & Ruoslahti, E. (1999). Integrin signaling. Science, 285(5430), 1028–1032.
Gobin, A. S., & West, J. L. (2002). Cell migration through defined, synthetic ECM analogs. FASEB Journal, 16(7), 751–753.
Goldmann, W. H. (2002). Mechanical aspects of cell shape regulation and signaling. Cell Biology International, 26(4), 313–317.
Gray, D. S., Tien, J., & Chen, C. S. (2003). Repositioning of cells by mechanotaxis on surfaces with micropatterned Young’s modulus. Journal of Biomedical Materials Research A, 66(3), 605–614.
Grinnell, F. (2000). Fibroblast-collagen-matrix contraction: Growth-factor signalling and mechanical loading. Trends in Cell Biology, 10(9), 362–365.
Grinnell, F. (2003). Fibroblast biology in three-dimensional collagen matrices. Trends in Cell Biology, 13(5), 264–269.
Gunn, J. W., Turner, S. D., & Mann, B. K. (2005). Adhesive and mechanical properties of hydrogels influence neurite extension. Journal of Biomedical Materials Research A, 72(1), 91–97.
Guo, W. H., Frey, M. T., Burnham, N. A., & Wang, Y. L. (2006). Substrate rigidity regulates the formation and maintenance of tissues. Biophysical Journal, 90(6), 2213–2220.
Hall, A. (1998). Rho GTPases and the actin cytoskeleton. Science, 279(5350), 509–514.
Halliday, N. L., & Tomasek, J. J. (1995). Mechanical properties of the extracellular matrix influence fibronectin fibril assembly in␣vitro. Experimental Cell Research, 217(1), 109–117.
Hanahan, D., & Weinberg, R. A. (2000). The hallmarks of cancer. Cell, 100(1), 57–70.
Harris, A. K., Wild, P., & Stopak, D. (1980). Silicone rubber substrata: A new wrinkle in the study of cell locomotion. Science, 208(4440), 177–179.
Hern, D. L., & Hubbell, J. A. (1998). Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. Journal of Biomedical Material Research, 39(2), 266–276.
Hu, S., Chen, J., Butler, J. P., & Wang, N. (2005). Prestress mediates force propagation into the nucleus. Biochemical and Biophysical Research Communication, 329(2), 423–428.
Hu, S., & Wang, N. (2006). Control of stress propagation in the cytoplasm by prestress and loading frequency. Molecular and Cellular Biomechanics, 3(2), 49–60.
Huang, S., & Ingber, D. E. (1999). The structural and mechanical complexity of cell-growth control. Nature Cell Biology, 1(5), E131–E138.
Hynes, R. O. (2002). Integrins: Bidirectional, allosteric signaling machines. Cell, 110(6), 673–687.
Ingber, D. E. (1997). Tensegrity: The architectural basis of cellular mechanotransduction. Annual Review of Physiology, 59, 575–599.
Ingber, D. E. (2002). Mechanical signaling and the cellular response to extracellular matrix in angiogenesis and cardiovascular physiology. Circulation Research, 91(10), 877–887.
Ingber, D. E. (2003). Tensegrity II. How structural networks influence cellular information processing networks. Journal of Cell Science, 116(Pt 8), 1397–1408.
Ingber, D. E. (2003). Tensegrity I. Cell structure and hierarchical systems biology. Journal of Cell Science, 116(Pt 7), 1157–1173.
Ingber, D. E. (2006). Cellular mechanotransduction: Putting all the pieces together again. FASEB Journal, 20(7), 811–827.
Ingber, D. E., & Folkman, J. (1989). Mechanochemical switching between growth and differentiation during fibroblast growth factor-stimulated angiogenesis in␣vitro: role of extracellular matrix. Journal of Cell Biology, 109(1), 317–330.
Ingber, D. E., Madri, J. A., & Jamieson, J. D. (1985). Neoplastic disorganization of pancreatic epithelial cell-cell relations. Role of basement membrane. American Journal of Pathology, 121(2), 248–260.
Isenberg, B. C., & Tranquillo, R. T. (2003). Long-term cyclic distention enhances the mechanical properties of collagen-based media-equivalents. Annals of Biomedical Engineering, 31(8), 937–949.
Jain, R. K. (2003). Molecular regulation of vessel maturation. Nature Medicine, 9(6), 685–693.
Kalluri, R., & Zeisberg, M. (2006). Fibroblasts in cancer. Nature Reviews Cancer, 6(5), 392–401.
Katsumi, A., Milanini, J., Kiosses, W. B., del Pozo, M. A., Kaunas, R., Chien, S., Hahn, K. M., & Schwartz, M. A. (2002). Effects of cell tension on the small GTPase Rac. Journal of Cell Biology, 158(1), 153–164.
Keeley, F. W., & Bartoszewicz, L. A. (1995). Elastin in systemic and pulmonary hypertension. Ciba Foundation Symposium, 192, 259–273; discussion 273–278.
Khademhosseini, A., Langer, R., Borenstein, J., & Vacanti, J. P. (2006). Microscale technologies for tissue engineering and biology. Proceedings of the National Academy of Sciences of the United States of America, 103(8), 2480–2487.
Khatiwala, C. B., Peyton, S. R., & Putnam, A. J. (2006). Intrinsic mechanical properties of the extracellular matrix affect the behavior of pre-osteoblastic MC3T3-E1 cells. American Journal of Physiology Cell Physiology, 290(6), C1640–C1650.
Kim, B. S., Nikolovski, J., Bonadio, J., & Mooney, D. J. (1999). Cyclic mechanical strain regulates the development of engineered smooth muscle tissue. Nature Biotechnology, 17(10), 979–983.
Kim, B. S., Nikolovski, J., Bonadio, J., Smiley, E., & Mooney, D. J. (1999). Engineered smooth muscle tissues: Regulating cell phenotype with the scaffold. Experimental Cell Research, 251(2), 318–328.
Kim, J. K., Xu, Y., Xu, X., Keene, D. R., Gurusiddappa, S., Liang, X., Wary, K. K., & Hook, M. (2005). A novel binding site in collagen type III for integrins alpha1beta1 and alpha2beta1. Journal of Biological Chemistry, 280(37), 32512–32520.
Kim, Y. B., Yu, J., Lee, S. Y., Lee, M. S., Ko, S. G., Ye, S. K., Jong, H. S., Kim, T. Y., Bang, Y. J., & Lee, J. W. (2005). Cell adhesion status-dependent histone acetylation is regulated through intracellular contractility-related signaling activities. Journal of Biological Chemistry, 280(31), 28357–28364.
Kleinman, H. K., & Martin, G. R. (2005). Matrigel: Basement membrane matrix with biological activity. Seminars in Cancer Biology, 15(5), 378–386.
Knight, C. G., Morton, L. F., Onley, D. J., Peachey, A. R., Messent, A. J., Smethurst, P. A., Tuckwell, D. S., Farndale, R. W., & Barnes, M. J. (1998). Identification in collagen type I of an integrin alpha2 beta1-binding site containing an essential GER sequence. Journal of Biological Chemistry, 273(50), 33287–33294.
Knight, C. G., Morton, L. F., Peachey, A. R., Tuckwell, D. S., Farndale, R. W., & Barnes, M. J. (2000). The collagen-binding A-domains of integrins alpha(1)beta(1) and alpha(2)beta(1) recognize the same specific amino acid sequence, GFOGER, in native (triple-helical) collagens. Journal of Biological Chemistry, 275(1), 35–40.
Kolodney, M. S., & Elson, E. L. (1995). Contraction due␣to␣microtubule disruption is associated with increased phosphorylation of myosin regulatory light chain. Proceedings of the National Academy of Sciences of the United States of America, 92(22), 10252–10256.
Kong, H. J., Wong, E., & Mooney, D. J. (2003). Independent control of rigidity and toughness of polymeric hydrogels. Macromolecules, 36(12), 4582–4588.
Korff, T., & Augustin, H. G. (1999). Tensional forces in fibrillar extracellular matrices control directional capillary sprouting. Journal of Cell Science, 112(Pt 19), 3249–3258.
Kumar, S., Maxwell, I. Z., Heisterkamp, A., Polte, T. R., Lele, T. P., Salanga, M., Mazur, E., & Ingber, D. E. (2006). Viscoelastic retraction of single living stress fibers and its impact on cell shape, cytoskeletal organization, and extracellular matrix mechanics. Biophysical Journal, 90(10), 3762–3773.
Langer, R., & Vacanti, J. P. (1993). Tissue engineering. Science, 260(5110), 920–926.
Lee, K. Y., & Mooney, D. J. (2001). Hydrogels for tissue engineering. Chemical Reviews, 101(7), 1869–1879.
Lehoux, S., Esposito, B., Merval, R., & Tedgui, A. (2005). Differential regulation of vascular focal adhesion kinase by steady stretch and pulsatility. Circulation, 111(5), 643–649.
Lehoux, S., & Tedgui, A. (1998). Signal transduction of mechanical stresses in the vascular wall. Hypertension, 32(2), 338–345.
Li, C., & Xu, Q. (2000). Mechanical stress-initiated signal transductions in vascular smooth muscle cells. Cell Signal, 12(7), 435–445.
Lin, Y. C., Ho, C. H., & Grinnell, F. (1998). Decreased PDGF receptor kinase activity in fibroblasts contracting stressed collagen matrices. Experimental Cell Research, 240(2), 377–387.
Liu, V. A., & Bhatia, S. N. (2002). Three-dimensional photopatterning of hydrogels containing living cells. Biomedical Microdevices, 4(4), 257–266.
Liu, B. P., Chrzanowska-Wodnicka, M., & Burridge, K. (1998). Microtubule depolymerization induces stress fibers, focal adhesions, and DNA synthesis via the GTP-binding protein Rho. Cell Adhesion and Communication, 5(4), 249–255.
Lo, C. M., Wang, H. B., Dembo, M., & Wang, Y. L. (2000). Cell movement is guided by the rigidity of the substrate. Biophysical Journal, 79(1), 144–152.
Maniotis, A. J., Chen, C. S., & Ingber, D. E. (1997). Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proceedings of the National Academy of Sciences of the United States of America, 94(3), 849–854.
Mann, B. K., Gobin, A. S., Tsai, A. T., Schmedlen, R. H., & West, J. L. (2001). Smooth muscle cell growth in photopolymerized hydrogels with cell adhesive and proteolytically degradable domains: synthetic ECM analogs for tissue engineering. Biomaterials, 22(22), 3045–3051.
Mann, B. K., & West, J. L. (2002). Cell adhesion peptides alter smooth muscle cell adhesion, proliferation, migration, and matrix protein synthesis on modified surfaces and in polymer scaffolds. Journal of Biomedical Material Research, 60(1), 86–93.
Martens, P. J., Bryant, S. J., & Anseth, K. S. (2003). Tailoring the degradation of hydrogels formed from multivinyl poly(ethylene glycol) and poly(vinyl alcohol) macromers for cartilage tissue engineering. Biomacromolecules, 4(2), 283–292.
Matthews, B. D., Overby, D. R., Mannix, R., & Ingber, D. E. (2006). Cellular adaptation to mechanical stress: Role of integrins, Rho, cytoskeletal tension and mechanosensitive ion channels. Journal of Cell Science, 119(Pt 3), 508–518.
McBeath, R., Pirone, D. M., Nelson, C. M., Bhadriraju, K., & Chen, C. S. (2004). Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental Cell, 6(4), 483–495.
Mooney, D., Hansen, L., Vacanti, J., Langer, R., Farmer, S., & Ingber, D. (1992). Switching from differentiation to growth in hepatocytes: Control by extracellular matrix. Journal of Cell Physiology, 151(3), 497–505.
Munevar, S., Wang, Y., & Dembo, M. (2001). Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophysical Journal, 80(4), 1744–1757.
Munevar, S., Wang, Y. L., & Dembo, M. (2001). Distinct roles of frontal and rear cell-substrate adhesions in fibroblast migration. Molecular Biology of the Cell, 12(12), 3947–3954.
Nehls, V., & Herrmann, R. (1996). The configuration of fibrin clots determines capillary morphogenesis and endothelial cell migration. Microvascular Research, 51(3), 347–364.
Netti, P. A., Berk, D. A., Swartz, M. A., Grodzinsky, A. J., & Jain, R. K. (2000). Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Research, 60(9), 2497–2503.
Nguyen, K. T., & West, J. L. (2002). Photopolymerizable hydrogels for tissue engineering applications. Biomaterials, 23(22), 4307–4314.
Nicolas, A., Geiger, B., & Safran, S. A. (2004). Cell mechanosensitivity controls the anisotropy of focal adhesions. Proceedings of the National Academy of Sciences of the United States of America, 101(34), 12520–12525.
Nikolovski, J., Kim, B. S., & Mooney, D. J. (2003). Cyclic strain inhibits switching of smooth muscle cells to an osteoblast-like phenotype. FASEB Journal, 17, 455–457.
Nobes, C. D., & Hall, A. (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell, 81(1), 53–62.
Numaguchi, K., Eguchi, S., Yamakawa, T., Motley, E. D., & Inagami, T. (1999). Mechanotransduction of rat aortic vascular smooth muscle cells requires RhoA and intact actin filaments. Circulation Research, 85(1), 5–11.
Ogut, O., & Brozovich, F. V. (2003). Regulation of force in vascular smooth muscle. Journal of Molecular Cellular Cardiology, 35(4), 347–355.
Oldberg, A., Franzen, A., & Heinegard, D. (1986). Cloning and sequence analysis of rat bone sialoprotein (osteopontin). cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proceedings of the National Academy of Sciences of the United States of America, 83(23), 8819–8823.
Opas, M. (1989). Expression of the differentiated phenotype by epithelial cells in␣vitro is regulated by both biochemistry and mechanics of the substratum. Developmental Biology, 131(2), 281–293.
Palazzo, A. F., Cook, T. A., Alberts, A. S., & Gundersen, G. G. (2001). mDia mediates Rho-regulated formation and orientation of stable microtubules. Nature Cell Biology, 3(8), 723–729.
Palecek, S. P., Loftus, J. C., Ginsberg, M. H., Lauffenburger, D. A., & Horwitz, A. F. (1997). Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature, 385(6616), 537–540.
Paszek, M. J., & Weaver, V. M. (2004). The tension mounts: mechanics meets morphogenesis and malignancy. Journal of Mammary Gland Biology and Neoplasia, 9(4), 325–342.
Paszek, M. J., Zahir, N., Johnson, K. R., Lakins, J. N., Rozenberg, G. I., Gefen, A., Reinhart-King, C. A., Margulies, S. S., Dembo, M., Boettiger, D., Hammer, D. A., & Weaver, V. M. (2005). Tensional homeostasis and the malignant phenotype. Cancer Cell, 8(3), 241–254.
Pelham, R. J. Jr., & Wang, Y. (1997). Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America, 94(25), 13661–13665.
Pelham, R. J. Jr., & Wang, Y. L. (1998). Cell locomotion and focal adhesions are regulated by the mechanical properties of the substrate. Biological Bulletin, 194(3), 348–349; discussion 349–350.
Petersen, O. W., Ronnov-Jessen, L., Howlett, A. R., & Bissell, M. J. (1992). Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proceedings of the National Academy of Sciences of the United States of America, 89(19), 9064–9068.
Petit, V., & Thiery, J. P. (2000). Focal adhesions: Structure and dynamics. Biologie Cellulaire, 92(7), 477–494.
Peyton, S. R., & Putnam, A. J. (2005). Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. Journal of Cell Physiology, 204(1), 198–209.
Peyton, S. R., Raub, C. B., Keschrumrus, V. P., & Putnam, A. J. (2006). The use of poly(ethylene glycol) hydrogels to investigate the impact of ECM chemistry and mechanics on smooth muscle cells. Biomaterials, 27(28), 4881–4893.
Pierschbacher, M. D., Ruoslahti, E., Sundelin, J., Lind, P., & Peterson, P. A. (1982). The cell attachment domain of fibronectin. Determination of the primary structure. Journal of Biological Chemistry, 257(16), 9593–9597.
Polte, T. R., Eichler, G. S., Wang, N., & Ingber, D. E. (2004). Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. American Journal of Physiology Cell Physiology, 286(3), C518–C528.
Powell, C. A., Smiley, B. L., Mills, J., & Vandenburgh, H. H. (2002). Mechanical stimulation improves tissue-engineered human skeletal muscle. American Journal of Physiology Cell Physiology, 283(5), C1557–C1565.
Putnam, A. J., Cunningham, J. J., Dennis, R. G., Linderman, J. J., & Mooney, D. J. (1998). Microtubule assembly is regulated by externally applied strain in cultured smooth muscle cells. Journal of Cell Science, 111(Pt 22), 3379–3387.
Putnam, A. J., Cunningham, J. J., Pillemer, B. B., & Mooney, D. J. (2003). External mechanical strain regulates membrane targeting of Rho GTPases by controlling microtubule assembly. American Journal of Physiology Cell Physiology, 284(3), C627–C639.
Putnam, A. J., Schultz, K., & Mooney, D. J. (2001). Control of microtubule assembly by extracellular matrix and externally applied strain. American Journal of Physiology Cell Physiology, 280(3), C556–C564.
Pytela, R., Pierschbacher, M. D., Argraves, S., Suzuki, S., & Ruoslahti, E. (1987). Arginine-glycine-aspartic acid adhesion receptors. Methods Enzymology, 144, 475–489.
Raeber, G. P., Lutolf, M. P., & Hubbell, J. A. (2005). Molecularly engineered PEG hydrogels: A novel model system for proteolytically mediated cell migration. Biophysical Journal, 89(2), 1374–1388.
Ratcliffe, A., & Niklason, L. E. (2002). Bioreactors and bioprocessing for tissue engineering. Annals of the New York Academy of Sciences, 961, 210–215.
Ren, X. D., Kiosses, W. B., & Schwartz, M. A. (1999). Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO Journal, 18(3), 578–585.
Reusch, P., Wagdy, H., Reusch, R., Wilson, E., & Ives, H. E. (1996). Mechanical strain increases smooth muscle and decreases nonmuscle myosin expression in rat vascular smooth muscle cells. Circulation Research, 79(5), 1046–1053.
Reyes, C. D., & Garcia, A. J. (2004). Alpha2beta1 integrin-specific collagen-mimetic surfaces supporting osteoblastic differentiation. Journal of Biomedical Materials Research A, 69(4), 591–600.
Rezania, A., & Healy, K. E. (1999). Integrin subunits responsible for adhesion of human osteoblast-like cells to biomimetic peptide surfaces. Journal of Orthopedic Research, 17(4), 615–623.
Rowley, J. A., Madlambayan, G., & Mooney, D. J. (1999). Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials, 20(1), 45–53.
Ruoslahti, E., & Pierschbacher, M. D. (1987). New perspectives in cell adhesion: RGD and integrins. Science, 238(4826), 491–497.
Sastry, S. K., & Burridge, K. (2000). Focal adhesions: A nexus for intracellular signaling and cytoskeletal dynamics. Experimental Cell Research, 261(1), 25–36.
Schoenwaelder, S. M., & Burridge, K. (1999). Bidirectional signaling between the cytoskeleton and integrins. Current Opinion in Cell Biology, 11(2), 274–286.
Shemesh, T., Geiger, B., Bershadsky, A. D., & Kozlov, M. M. (2005). Focal adhesions as mechanosensors: A physical mechanism. Proceedings of the National Academy of Sciences of the United States of America, 102(35), 12383–12388.
Shyy, J. Y., & Chien, S. (1997). Role of integrins in cellular responses to mechanical stress and adhesion. Current Opinion in Cell Biology, 9(5), 707–713.
Shyy, J. Y., & Chien, S. (2002). Role of integrins in endothelial mechanosensing of shear stress. Circulation Research, 91(9), 769–775.
Sieminski, A. L., Hebbel, R. P., & Gooch, K. J. (2004). The relative magnitudes of endothelial force generation and matrix stiffness modulate capillary morphogenesis in␣vitro. Experimental Cell Research, 297(2), 574–584.
Sikavitsas, V. I., Temenoff, J. S., & Mikos, A. G. (2001). Biomaterials and bone mechanotransduction. Biomaterials, 22(19), 2581–2593.
Sordella, R., Jiang, W., Chen, G. C., Curto, M., & Settleman, J. (2003). Modulation of Rho GTPase signaling regulates a switch between adipogenesis and myogenesis. Cell, 113(2), 147–158.
Staatz, W. D., Fok, K. F., Zutter, M. M., Adams, S. P., Rodriguez, B. A., & Santoro, S. A. (1991). Identification of a tetrapeptide recognition sequence for the alpha 2 beta 1 integrin in collagen. Journal of Biological Chemistry, 266(12), 7363–7367.
Stamenovic, D., Fredberg, J. J., Wang, N., Butler, J. P., & Ingber, D. E. (1996). A microstructural approach to cytoskeletal mechanics based on tensegrity. Journal of Theoretical Biology, 181(2), 125–136.
Stamenovic, D., & Ingber, D. E. (2002). Models of cytoskeletal mechanics of adherent cells. Biomechanics and Modeling in Mechanobiology, 1(1), 95–108.
Stamenovic, D., Mijailovich, S. M., Tolic-Norrelykke, I. M., Chen, J., & Wang, N. (2002). Cell prestress. II. Contribution of microtubules. American Journal of Physiology Cell Physiology, 282(3), C617–C624.
Stegemann, J. P., Hong, H., & Nerem, R. M. (2005). Mechanical, biochemical, and extracellular matrix effects on vascular smooth muscle cell phenotype. Journal of Applied Physiology, 98(6), 2321–2327.
Stegemann, J. P., & Nerem, R. M. (2003). Phenotype modulation in vascular tissue engineering using biochemical and mechanical stimulation. Annals of Biomedical Engineering, 31(4), 391–402.
Thyberg, J. (1998). Phenotypic modulation of smooth muscle cells during formation of neointimal thickenings following vascular injury. Histology and Histopathology, 13(3), 871–891.
Thyberg, J., Blomgren, K., Hedin, U., & Dryjski, M. (1995). Phenotypic modulation of smooth muscle cells during the formation of neointimal thickenings in the rat carotid artery after balloon injury: an electron-microscopic and stereological study. Cell Tissue Research, 281(3), 421–433.
Turner, C. H., & Pavalko, F. M. (1998). Mechanotransduction and functional response of the skeleton to physical stress: The mechanisms and mechanics of bone adaptation. Journal of Orthopedic Science, 3(6), 346–355.
Urech, L., Bittermann, A. G., Hubbell, J. A., & Hall, H. (2005). Mechanical properties, proteolytic degradability and biological modifications affect angiogenic process extension into native and modified fibrin matrices in␣vitro. Biomaterials, 26(12), 1369–1379.
Vailhe, B., Lecomte, M., Wiernsperger, N., & Tranqui, L. (1998). The formation of tubular structures by endothelial cells is under the control of fibrinolysis and mechanical factors. Angiogenesis, 2(4), 331–344.
Walker, G. A., Masters, K. S., Shah, D. N., Anseth, K. S., & Leinwand, L. A. (2004). Valvular myofibroblast activation by transforming growth factor-beta: Implications for pathological extracellular matrix remodeling in heart valve disease. Circulation Research, 95(3), 253–260.
Wang, Y., Botvinick, E. L., Zhao, Y., Berns, M. W., Usami, S., Tsien, R. Y., & Chien, S. (2005). Visualizing the mechanical activation of Src. Nature, 434(7036), 1040–1045.
Wang, N., Butler, J. P., & Ingber, D. E. (1993). Mechanotransduction across the cell surface and through the cytoskeleton. Science, 260(5111), 1124–1127.
Wang, H. B., Dembo, M., Hanks, S. K., & Wang, Y. (2001). Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proceedings of the National Academy of Sciences of the United States of America, 98(20), 11295–11300.
Wang, N., Naruse, K., Stamenovic, D., Fredberg, J. J., Mijailovich, S. M., Tolic-Norrelykke, I. M., Polte, T., Mannix, R., & Ingber, D. E. (2001). Mechanical behavior in living cells consistent with the tensegrity model. Proceedings of the National Academy of Sciences of the United States of America, 98(14), 7765–7770.
Wang, N., & Stamenovic, D. (2002). Mechanics of vimentin intermediate filaments. Journal of Muscle Research and Cell Motility, 23(5–6), 535–540.
Wang, N., & Suo, Z. (2005). Long-distance propagation of forces in a cell. Biochemical and Biophysical Research Communication, 328(4), 1133–1138.
Wang, N., Tolic-Norrelykke, I. M., Chen, J., Mijailovich, S. M., Butler, J. P., Fredberg, J. J., & Stamenovic, D. (2002). Cell prestress. I. Stiffness and prestress are closely associated in adherent contractile cells. American Journal of Physiology Cell Physiology, 282(3), C606–C616.
Wang, F., Weaver, V. M., Petersen, O. W., Larabell, C. A., Dedhar, S., Briand, P., Lupu, R., & Bissell, M. J. (1998). Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proceedings of the National Academy of Sciences of the United States of America, 95(25), 14821–14826.
Weaver, V. M., Petersen, O. W., Wang, F., Larabell, C. A., Briand, P., Damsky, C., & Bissell, M. J. (1997). Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in␣vivo by integrin blocking antibodies. Journal of Cell Biology, 137(1), 231–245.
Wehrle-Haller, B., & Imhof, B. (2002). The inner lives of focal adhesions. Trends in Cell Biology, 12(8), 382–389.
Wilson, E., Sudhir, K. & Ives, H. E. (1995). Mechanical strain of rat vascular smooth muscle cells is sensed by specific extracellular matrix/integrin interactions. Journal of Clinical Investigation, 96(5), 2364–2372.
Wittmann, T., & Waterman-Storer, C. M. (2001). Cell motility: Can Rho GTPases and microtubules point the way?. Journal of Cell Science, 114(Pt 21), 3795–3803.
Wolff, J. (1992). Das Gasetz der Transformation der Knochen. Berlin: Verlag August Hirschwald.
Wong, J. Y., Velasco, A., Rajagopalan, P., & Pham, Q. (2003). Directed movement of vascular smooth muscle cells on gradient-compliant hydrogels. Langmuir, 19(5), 1908–1913.
Wozniak, M. A., Desai, R., Solski, P. A., Der, C. J., & Keely, P. J. (2003). ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. Journal of Cell Biology, 163(3), 583–595.
Wozniak, M. A., Modzelewska, K., Kwong, L., & Keely, P. J. (2004). Focal adhesion regulation of cell behavior. Biochimica et Biophysica Acta, 1692(2–3), 103–119.
Yeung, T., Georges, P. C., Flanagan, L. A., Marg, B., Ortiz, M., Funaki, M., Zahir, N., Ming, W., Weaver, V., & Janmey, P. A. (2005). Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motility and the Cytoskeleton, 60(1), 24–34.
Zaari, N., Rajagopalan, P., Kim, S. K., Engler, A. J., & Wong, J. Y. (2004). Photopolymerization in microfluidic gradient generators: Microscale control of substrate compliance to manipulate cell response. Advanced Materials, 16(23–24), 2133.
Zaman, M. H., Kamm, R. D., Matsudaira, P., & Lauffenburger, D. A. (2005). Computational model for cell migration in three-dimensional matrices. Biophysical Journal, 89(2), 1389–1397.
Zaman, M. H., Trapani, L. M., Siemeski, A., Mackellar, D., Gong, H., Kamm, R. D., Wells, A., Lauffenburger, D. A., & Matsudaira, P. (2006). Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proceedings of the National Academy of Sciences of the United States of America, 103(29), 10889–10894.
Zhang, Q., Magnusson, M. K., & Mosher, D. F. (1997). Lysophosphatidic acid and microtubule-destabilizing agents stimulate fibronectin matrix assembly through Rho-dependent actin stress fiber formation and cell contraction. Molecular Biology of the Cell, 8(8), 1415–1425.
Zhong, C., Chrzanowska-Wodnicka, M., Brown, J., Shaub, A., Belkin, A. M., & Burridge, K. (1998). Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. Journal of Cell Biology, 141(2), 539–551.
Acknowledgments
The authors gratefully acknowledge financial support from the National Institutes of Health (NIDCR: DE-016117) and the American Heart Association (Western States Affiliate: 0465111Y) to A.J.P. and fellowships from the Achievement Rewards for College Scientists (ARCS) Foundation to S.R.P. and C.M.G.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Peyton, S.R., Ghajar, C.M., Khatiwala, C.B. et al. The emergence of ECM mechanics and cytoskeletal tension as important regulators of cell function. Cell Biochem Biophys 47, 300–320 (2007). https://doi.org/10.1007/s12013-007-0004-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-007-0004-y