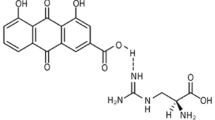
Abstract
Diabetes‐induced endothelial dysfunction is critical for the development of diabetic cardiovascular complications. The aim of this study was to investigate the effect of niclosamide (Nic) on vascular endothelial dysfunction in streptozotocin (STZ)-induced diabetic rats. Male Sprague–Dawley rats were injected with a single intraperitoneal injection of STZ (75 mg/kg) to induce type 1 diabetes, and Nic (10 mg/kg) was intraperitoneally administered per day for 4 weeks. Endothelial function was evaluated as carbachol (CCh, an endothelium-dependent vasodilator)-evoked relaxation in the experiments performed on isolated thoracic aortas. The changes in the protein expressions of phosphorylated eNOS at serine 1177 (p-eNOSSer1177) and phosphorylated VASP at serine 239 (p-VASPSer239) of the rat aortas were analyzed by western blotting to determine whether NO/cGMP signaling is involved in the mechanism of Nic. STZ-injected rats had higher fasting blood glucose and less body weight compared to control rats (p < 0.05). Nic treatment did not affect blood glucose levels or body weights of the rats. CCh-induced endothelium-dependent relaxation of the aortic rings was significantly decreased in diabetic rats compared to control (Emax = 66.79 ± 7.41% and 90.28 ± 5.55%, respectively; p < 0.05). CCh-induced relaxation response was greater in Nic-treated diabetic rats compared to diabetic rats (Emax = 91.56 ± 1.20% and 66.79 ± 7.41%, respectively; p < 0.05). Phosphorylation of eNOS and VASP in aortic tissues was significantly reduced in diabetic rats, which were markedly increased by Nic treatment (p < 0.05). We demonstrated that Nic improved endothelial dysfunction possibly through the activation of NO/cGMP signaling without affecting hyperglycemia in diabetic rats. Our results suggesting that Nic has potential of repurposing for diabetic cardiovascular complications.




Similar content being viewed by others
References
Skyler, J. S., Bakris, G. L., Bonifacio, E., Darsow, T., Eckel, R. H., Groop, L., et al. (2017). Differentiation of diabetes by pathophysiology, natural history, and prognosis. Diabetes, 66(2), 241–255.
Saeedi, P., Petersohn, I., Salpea, P., Malanda, B., Karuranga, S., Unwin, N., et al. (2019). Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes research and clinical practice, 157, 107843.
Chowdhury, T. A., Shaho, S., & Moolla, A. (2014). Complications of diabetes: Progress, but significant challenges ahead. Annals of Translational Medicine, 2(12), 120.
Leon, B. M., & Maddox, T. M. (2015). Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World Journal of Diabetes, 6(13), 1246–1258.
Jia, X., Xiao, C., Sheng, D., et al. (2020). TRPV4 Mediates cardiac fibrosis via the TGF-β1/Smad3 signaling pathway in diabetic rats. Cardiovascular Toxicology, 20, 492–499.
Zeydanli, E. N., Kandilci, H. B., & Turan, B. (2011). Doxycycline ameliorates vascular endothelial and contractile dysfunction in the thoracic aorta of diabetic rats. Cardiovascular Toxicology, 11, 134–147.
Sena, C. M., Pereira, A. M., & Seiça, R. (2013). Endothelial dysfunction—A major mediator of diabetic vascular disease. Biochimica et Biophysica Acta (BBA)—Molecular Basis of Disease, 1832(32), 2216–2232.
Pooja, G. D., Bhargava, K., & Sethy, N. K. (2018). Post-translational modifications of eNOS augment nitric oxide availability and facilitates hypoxia adaptation in Ladakhi women. Nitric Oxide, 78, 103–112.
Park, M., Sandner, P., & Krieg, T. (2018). cGMP at the centre of attention: Emerging strategies for activating the cardioprotective PKG pathway. Basic Research in Cardiology, 113(4), 1–7.
Du, X., Chen, C., Zhang, M., Cai, D., Sun, J., Yang, J., et al. (2015). Scutellarin reduces endothelium dysfunction through the PKG-I pathway. Journal of Evidence-Based Integrative Medicine, 2015, 430271–430312.
Oelze, M., Mollnau, H., Hoffmann, N., Warnholtz, A., Bodenschatz, M., Smolenski, A., et al. (2000). Vasodilator-stimulated phosphoprotein serine 239 phosphorylation as a sensitive monitor of defective nitric oxide/cGMP signaling and endothelial dysfunction. Circulation Research, 87(11), 999–1005.
Matsumoto, T., Noguchi, E., Kobayashi, T., & Kamata, K. (2007). Mechanisms underlying the chronic pioglitazone treatment-induced improvement in the impaired endothelium-dependent relaxation seen in aortas from diabetic rats. Free Radical Biology and Medicine, 42(7), 993–1007.
Wang, Z., Wu, Y., Zhang, S., Zhao, Y., Yin, X., Wang, W., et al. (2019). The role of NO-cGMP pathway inhibition in vascular endothelial-dependent smooth muscle relaxation disorder of AT1-AA positive rats: Protective effects of adiponectin. Nitric Oxide, 87, 10–22.
Chen, W., Mook, R. A., Jr., Premont, R. T., & Wang, J. (2018). Niclosamide: Beyond an antihelminthic drug. Cellular Signalling, 41, 89–96.
Li, Y., Li, P. K., Roberts, M. J., Arend, R. C., Samant, R. S., & Buchsbaum, D. J. (2014). Multi-targeted therapy of cancer by niclosamide: A new application for an old drug. Cancer Letters, 349(1), 8–14.
Han, P., Shao, M., Guo, L., Wang, W., Song, G., Yu, X., et al. (2018). Niclosamide ethanolamine improves diabetes and diabetic kidney disease in mice. American Journal of Translational Research, 10(4), 1071–1084.
Li, S. L., Yan, J., Zhang, Y. Q., Zhen, C. L., Liu, M. Y., Jin, J., et al. (2017). Niclosamide ethanolamine inhibits artery constriction. Pharmacological Research, 115, 78–86.
Sezen, S. F., Lagoda, G., Musicki, B., & Burnett, A. L. (2014). Hydroxyl fasudil, an inhibitor of Rho signaling, improves erectile function in diabetic rats: A role for neuronal ROCK. The Journal of Sexual Medicine, 11(9), 2164–2171.
Elbe, H., Vardı, N., Orman, D., Taşlıdere, E., & Yıldız, A. (2014). Ameliorative effects of aminoguanidine on rat aorta in streptozotocin-induced diabetes and evaluation of α-SMA expression. Anatolian Journal of Cardiology, 14(8), 679–684.
Kajal, A., & Singh, R. (2019). Coriandrum sativum seeds extract mitigate progression of diabetic nephropathy in experimental rats via AGEs inhibition. PLoS ONE, 14(3), e0213147.
Ghareib, S. A., El-Bassossy, H. M., Elberry, A. A., Azhar, A., Watson, M. L., Banjar, Z. M., et al. (2016). Protective effect of zingerone on increased vascular contractility in diabetic rat aorta. European Journal of Pharmacology, 780, 174–179.
Kadioglu, M., Kaya Yasar, Y., Barut, E. N., & Engin, S. (2019). Trimebutine maleate relaxes the isolated rat thoracic aorta: The role of nitric oxide and L-type calcium channels. Clinical and Experimental Pharmacology and Physiology, 46, 322–328.
Cicek, F. A., Kandilci, H. B., & Turan, B. (2013). Role of ROCK upregulation in endothelial and smooth muscle vascular functions in diabetic rat aorta. Cardiovascular Diabetology, 12, 51.
Leng, B., Tang, F., Lu, M., Zhang, Z., Wang, H., & Zhang, Y. (2018). Astragaloside IV improves vascular endothelial dysfunction by inhibiting the TLR4/NF-κB signaling pathway. Life Sciences, 209, 111–121.
Aronson, D., & Edelman, E. R. (2014). Coronary artery disease and diabetes mellitus. Cardiology Clinics, 32(3), 439–455.
van Dieren, S., Beulens, J. W., van der Schouw, Y. T., Grobbee, D. E., & Neal, B. (2010). Theglobal burden of diabetes its complications: An emerging pandemic. European Journal of Cardiovascular Prevention and Rehabilitation, 17(1), 3–8.
Rodriguez-Araujo, G., & Nakagami, H. (2018). Pathophysiology of cardiovascular disease in diabetes mellitus. Cardiovascular Endocrinology and Metabolism, 7(1), 4–9.
Bakker, W., Eringa, E. C., Sipkema, P., & van Hinsbergh, V. W. M. (2009). Endothelial dysfunction and diabetes: Roles of hyperglycemia, impaired insulin signaling and obesity. Cell and Tissue Research, 335, 165–189.
Tabit, C. E., Chung, W. B., Hamburg, N. M., & Vita, J. A. (2010). Endothelial dysfunction in diabetes mellitus: Molecular mechanisms and clinical implications. Reviews in Endocrine and Metabolic Disorders, 11(1), 61–74.
Kukreja, R. C., & Xi, L. (2007). eNOS phosphorylation: A pivotal molecular switch in vasodilation and cardioprotection? Journal of Molecular and Cellular Cardiology, 42(2), 280–282.
Davis, B. J., Xie, Z., Viollet, B., & Zou, M. H. (2006). Activation of the AMP-activated kinase by antidiabetes drug metformin stimulates nitric oxide synthesis in vivo by promoting the association of heat shock protein 90 and endothelial nitric oxide synthase. Diabetes, 55(2), 496–505.
Erdogdu, O., Nathanson, D., Sjöholm, A., Nyström, T., & Zhang, Q. (2010). Exendin-4 stimulates proliferation of human coronary artery endothelial cells through eNOS-, PKA- and PI3K/Akt-dependent pathways and requires GLP-1 receptor. Molecular and Cellular Endocrinology, 325(1–2), 26–35.
van den Oever, I. A., Raterman, H. G., Nurmohamed, M. T., & Simsek, S. (2010). Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators of Inflammation, 2010, 792393.
Faries, P. L., Rohan, D. I., Takahara, H., Wyers, M. C., Contreras, M. A., Quist, W. C., et al. (2001). Human vascular smooth muscle cells of diabetic origin exhibit increased proliferation, adhesion, and migration. Journal of Vascular Surgery, 33(3), 601–607.
Huang, M., Qiu, Q., Zeng, S., Xiao, Y., Shi, M., Zou, Y., et al. (2015). Niclosamide inhibits the inflammatory and angiogenic activation of human umbilical vein endothelial cells. Inflammation research: Official journal of the European Histamine Research Society, 64(12), 1023–1032.
Xiao, X. L., Hu, N., Zhang, X. Z., Jiang, M., Chen, C., Ma, R., et al. (2018). Niclosamide inhibits vascular smooth muscle cell proliferation and migration and attenuates neointimal hyperplasia in injured rat carotid arteries. British Journal of Pharmacology, 175(10), 1707–1718.
Khodadadi, S., Zabihi, N. A., Niazmand, S., Abbasnezhad, A., Mahmoudabady, M., & Rezaee, S. A. (2018). Teucrium polium improves endothelial dysfunction by regulating eNOS and VCAM-1 genes expression and vasoreactivity in diabetic rat aorta. Biomedicine and Pharmacotherapy, 103, 1526–1530.
Taguchi, K., Narimatsu, H., Matsumoto, T., & Kobayashi, T. (2019). ERK-containing microparticles from a diabetic mouse induce endothelial dysfunction. Journal of Endocrinology, 241(3), 221–233.
Coban, O., Aytac, Z., Yildiz, Z. I., & Uyar, T. (2020). Colon targeted delivery of niclosamide from β-cyclodextrin inclusion complex incorporated electrospun Eudragit® L100 nanofibers. Colloids and Surfaces B: Biointerfaces, 197, 111391.
Xu, N., Wang, Q., Jiang, S., Wang, Q., Hu, W., Zhou, S., et al. (2019). Fenofibrate improves vascular endothelial function and contractility in diabetic mice. Redox Biology, 20, 87–97.
Tao, H., Zhang, Y., Zeng, X., Shulman, G. I., & Jin, S. (2014). Niclosamide ethanolamine-induced mild mitochondrial uncoupling improves diabetic symptoms in mice. Nature Medicine, 11, 1263–1269.
Suzuki, T., Kojima, M., Matsumoto, Y., Kobayashi, K. I., Inoue, J., & Yamamoto, Y. (2020). Niclosamide activates the AMP-activated protein kinase complex containing the β2 subunit independently of AMP. Biochemical and Biophysical Research Communications, 533(4), 758–763.
Taguchi, K., Tano, I., Kaneko, N., Matsumoto, T., & Kobayashi, T. (2020). Plant polyphenols Morin and Quercetin rescue nitric oxide production in diabetic mouse aorta through distinct pathways. Biomedicine and Pharmacotherapy, 129, 110463.
Acknowledgement
This study supported by the grants from the Scientific Research Project Coordination Unit of Karadeniz Technical University (Project No. TDK-2018-7328).
Author information
Authors and Affiliations
Contributions
Concept—SE; design—SE, YKY; supervision—SFS; materials—SE, YKY, and ENB, data collection and/or processing—SE, YKY, and ENB; analysis and/or interpretation—SE and YKY; literature search—SE and YKY; writing—SE and SFS; critical review—SE, YKY, ENB, and SFS.
Corresponding author
Ethics declarations
Conflict of ınterest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Handling Editor: Travis Knuckles.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Engin, S., Yasar, Y.K., Barut, E.N. et al. Improved Endothelium-Dependent Relaxation of Thoracic Aorta in Niclosamide-Treated Diabetic Rats. Cardiovasc Toxicol 21, 563–571 (2021). https://doi.org/10.1007/s12012-021-09647-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-021-09647-0