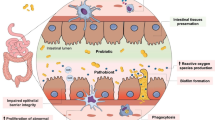
Abstract
Tight junctions (TJs) are the key determinant of barrier function in the mammary gland, with their disruption being associated with the pathogenesis and progression of mastitis, especially in the case of Staphylococcus aureus (S. aureus) infection. This study investigated whether selenium (Se) could attenuate S. aureus-induced mastitis by inhibiting inflammation and protecting mammary gland TJs in mice. The expression profiles of S. aureus-infected gland tissues derived from the gene expression omnibus dataset were analyzed. We found cytokine production, cell junctions, the nuclear transcription factor-κB (NF-κB) signalling pathway, and inflammatory responses associated with the differentially expressed genes, as revealed by Gene Ontology (GO) and Kyoto Encyclopaedia of Genes and Genomes (KEGG) enrichment analyses. Se reduced the mRNA expression and production of inflammatory cytokines, including tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6), and decreased phosphorylation levels of the NF-κB complex. Moreover, Se alleviated structural damage and microvillus injury in mammary glands. Immunohistochemical staining revealed that Se increased the expression of Claudin-3; Western blot analysis revealed increased protein levels of Occludin and Tricellulin in the group supplemented with dietary Se. In summary, Se counteracted TJ disruption and attenuated NF-κB-mediated inflammatory responses in S. aureus-infected mouse mammary glands.







Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article.
References
Kobayashi K (2023) Culture models to investigate mechanisms of milk production and blood-milk barrier in mammary epithelial cells: a review and a protocol. J Mammary Gland Biol Neoplasia 28(1):8
Tsugami Y, Nii T, Isobe N (2023) Valine treatment enhances antimicrobial component production in mammary epithelial cells and the milk of lactating goats without influencing the tight junction barrier. J Mammary Gland Biol Neoplasia 28(1):3
Nerger BA, Nelson CM (2019) 3D culture models for studying branching morphogenesis in the mammary gland and mammalian lung. Biomaterials 198:135–145
Caron TJ, Scott KE, Fox JG et al (2015) Tight junction disruption: Helicobacter pylori and dysregulation of the gastric mucosal barrier. World J Gastroenterol 21(40):11411–11427
Zheng Y, Zhao Y, He W et al (2022) Novel organic selenium source hydroxy-selenomethionine counteracts the blood-milk barrier disruption and inflammatory response of mice under heat stress. Front Immunol 13:1054128
Bi CL, Wang H, Wang YJ et al (2016) Selenium inhibits Staphylococcus aureus-induced inflammation by suppressing the activation of the NF-κB and MAPK signaling pathways in RAW264.7 macrophages. Eur J Pharmacol 780:159–165. https://doi.org/10.1016/j.ejphar.2016.03.044
Bi CL, Zhang SJ, Shen YZ et al (2021) Selenium plays an anti-inflammatory role by regulation NLRP3 inflammasome in Staphylococcue aureus-infected mouse mammary gland. Biol Trace Elem Res 199:604–610
Liu J, Wang X, Bi C et al (2022) Molecular characterization of multi-drug-resistant Staphylococcus aureus in mastitis bovine milk from a dairy farm in Anhui. China Front Vet Sci 9:966533
Morales-Ubaldo AL, Rivero-Perez N, Valladares-Carranza B et al (2023) Bovine mastitis, a worldwide impact disease: prevalence, antimicrobial resistance, and viable alternative approaches. Vet Anim Sci 21:100306
Chandler RL (1973) Ultrastructural and associated studies on experimental mastitis in the mouse produced by three strains of streptococcus. Br J Exp Pathol 54(3):267–273
Notebaert S, Meyer E (2006) Mouse models to study the pathogenesis and control of bovine mastitis. A Rev Vet Q 28(1):2–13
Wang Y, Lin Y, Cheng C et al (2020) NF-κB/TWIST1 mediates migration and phagocytosis of macrophages in the mice model of implant-associated Staphylococcus aureus osteomyelitis. Front Microbiol 11:1301
Mehdi Y, Dufrasne I (2016) Selenium in cattle: a review. Molecules 21(4):545
Cui L, Zhang J, Guo J et al (2023) Selenium suppressed the LPS-induced inflammation of bovine endometrial epithelial cells through NF-κB and MAPK pathways under high cortisol background. J Cell Mol Med 27(10):1373–1383
Wang JJ, Wei ZK, Zhang X et al (2017) Butyrate protects against disruption of the blood-milk barrier and moderates inflammatory responses in a model of mastitis induced by lipopolysaccharide. Br J Pharmacol 174(21):3811–3822
Shamir ER, Ewald AJ (2015) Adhesion in mammary development: novel roles for E-cadherin in individual and collective cell migration. Curr Top Dev Biol 112:353–382
Jaswal S, Jena MK, Anand V et al (2022) Critical review on physiological and molecular features during bovine mammary gland development: recent Advances. Cells 11(20):3325
Finot L, Chanat E, Dessauge F (2021) Mammary gland 3D cell culture systems in farm animals. Vet Res 52(1):78
Fiorentino M, Sapone A, Senger S et al (2016) Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism 7:49
Vermette D, Hu P, Canarie MF, Funaro M, Glover J, Pierce RW (2018) Tight junction structure, function, and assessment in the critically ill: a systematic review. Intensive Care Med Exp 6(1):37
Baumgartner HK, Rudolph MC, Ramanathan P et al (2017) Developmental expression of claudins in the mammary gland. J Mammary Gland Biol Neoplasia 22(2):141–157
Bhat AA, Uppada S, Achkar IW et al (2019) Tight junction proteins and signaling pathways in cancer and inflammation: a functional crosstalk. Front Physiol 9:1942
Funding
This work was supported by the Hebei Province Graduate Innovation Funding Project (CXZZBS2023075), The Major Basic Program of Natural Science Foundation of Shandong Province, China (ZR2019ZD21), and the project of Shandong Province Higher Educational Outstanding Youth Innovation Team (2019KJF011).
Author information
Authors and Affiliations
Contributions
Chong-Liang Bi contributed to the overall study design and supervised all research. Junjun Liu carried out the experiments, analyzed the data, and was also responsible for the final editing of the manuscript. Juan Wang contributed partly to the writing and finally revising the manuscript and data analysis. Shiyang Xv drafted and revised the first version of the manuscript. All the authors reviewed and finally approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
All the procedures of the study were performed under the approval of Laboratory Animals Research Center of Linyi Hebei province in P. R. China and the Ethics Committee of Linyi University and Hebei Agricultural University.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Junjun Liu and Juan Wang contributed equally to this work.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Wang, J., XV, S. et al. Selenium Counteracts Tight Junction Disruption and Attenuates the NF-κB-Mediated Inflammatory Response in Staphylococcus aureus-Infected Mouse Mammary Glands. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04210-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04210-8