Abstract
In this study, the moisture and biogenic element quantities of most wild and cultivated edible dried fruits were investigated. Macroelements found in highest amounts in fruits were K, P, Ca, and Mg. While K quantities of the fruits are recorded between 5212.77 (“white myrtle”) and 25550.60 mg/kg (“black nightshade”), P amounts of the fruits were characterized to be between 949.08 (black myrtle) and 4420.75 mg/kg (“black nightshade”). Ca and Mg amounts of the fruits were assessed to be between 359.83 (plum) and 4330.89 mg/kg (“yellow hawthorn”) to 214.98 (plum) and 1852.04 mg/kg (“black nightshade”), respectively. Fe and B quantities of the fruitss were established to be between 2.69 (“black myrtle”) and 60.13 mg/kg (cherry) to 3.76 (“black myrtle”) and 76.25 mg/kg (sour cherry), respectively. In general, except for white and black myrtle fruits, it is thought that other fruits can be good sources of P, K, and Fe. P contents of “laurel cherry,” “cherry,” “black nightshade,” and “Fragrant black grapes” were found partly high than those of other fruits. “Cherry laurel,” “sour cherry,” “cherry,” “pear,” “black nightshade,” “black fig,” and “hawthorn (yellow)” fruits contain more K than other fruits.
Graphical Abstract
In this study, the degree of accumulation of macro-, micro element contents of some wild and culktivated fruits was investigated.

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fruits, which are important sources of nutrients, are often known as a good source of nutrients and food supplements in the world [13, 15]. Mineral ions are very important for human health, and potassium, calcium, and magnesium, found in the tissues of many fruits, have been reported to be an important factor determining the quality of fruit storage [3, 10, 16]. Fruits are rich in various fat-free components such as vegetable protein, fiber, nutrients, and bioactive compounds [26]. Dried fruits, which have high levels of antioxidants, vitamins, essential amino acids, nutrients, and phytochemicals, are very beneficial for human health [11, 12, 14, 18, 24]. Dried fruits have many medicinal properties because they contain plenty of nutrient compounds, and they should always be included in our diet for their health benefits [5]. The mineral content of a given food item has been reported to be an important factor characterizing the nutritional quality of the food product [21]. The main factors affecting the availability of mineral elements in the soil are soil pH, cation exchange capacity, activity of microbes, organic matter, and water content [7, 8]. The gradual increase in industrial and agricultural activities since the beginning of the twentieth century and the development of technologies accordingly have brought about some problems such as environmental pollution and deterioration of the world ecosystem balance, thus causing increasing contamination of foodstuffs [23, 9]. This situation has also threatened the health of all living creatures and endangered the sustainability of the ecological environment. In recent years, heavy metals have emerged as one of the most important food contaminants that threaten human health [25]. Since a large-scale study determining the biogenic element contents of dried fruits is rare, the necessary amounts of these elements must be in the human diet to maintain a healthy life [19]. It can be said that these fruits, which have high nutritional value and important usage areas, are not known and do not receive the necessary attention in many regions of our country. Products such as pulp, nectar, marmalade, tea, and fruit extract can be obtained from these wild fruit species. In addition to enriching other fruit and vegetable juices with vitamins, it can also be used as a filling material in the cake and confectionery industry [4]. The aim of this research was to determine the macro and micro element contents of wild and cultivated edible fruits obtained from 4 different locations after drying.
Material and Methods
Material
Fruit samples were provided from Düzce (“Cherry Laurel,” “Fragrant Black Grapes,” “Strawberry Tree,” “Hawthorn (Yellow),” “Japonica Pear”), Adapazarı (“Fragrant black Nightshade,” “Kaki Persimmon,” “Black Fig,” “Damask Plum”), Isparta (“Sour Cherry,” “Cherry,” “Elaeagnus”), and Mersin (“White and black myrtles”) provinces in 2023 (Table 1). These fruits were preferred because they were more abundant in these locations. After the fruits were collected, they were transported to the laboratory in cold bags. Before the analysis, the fruits were washed with distilled water, and then the dried in a shade place. The environmental conditions are 68%, 32 °C, and 1100 m for average humidity, temperature, and altitude, respectively. HNO3 and H2O2 are analytical grade and Merck company (Darmstadt, Germany). The places where the fruits used in this study were collected are shown on the map below (Fig. 1).
Method
Determination of Moisture
The moisture amounts of the fruits were assigned at 70 °C/48 h using an oven till a constant weight [2].
Macro and Micro- Element Contents of Fruit Samples
After 0.2 g ground fruit samples (0.5 meh) were inginerated in a microwave device at 210 °C and 200 PSI pressure /45 min in 5 ml of concentrated HNO3 and 2 ml of H2O2 (30% w/v), the volumes of the dissolved samples were completed to 20 ml with deionized water. Then, heavy metal concentrations in the samples were analyzed with Inductively coupled plasma-optical emission spectrometry (ICP-OES) [24].
Working Conditions of ICP-OES
Its RF power of ICP-OES changes between 0.7 and 1.5 kw (1.2–1.3 kw for axial). In addition, plasma gas flow rate (Ar) ranged from 10.5 to 15 L/min (radial) 15″ (axial). Auxilary gas flow rate (Ar) is 1.5″. Viewing height is between 5 and 12 mm. Copy and reading time change between 1 and 5 s (max. 60 s).
Statistical Analysis
The JMP statistical program was used for the statistical analysis of results obtained. Statistically differences were determined by the analysis of variance (ANOVA) procedure in all data (p < 0.01) [22]. In order to examine the correlation between nutrient element contents of fruit types, a multivariate cluster analysis was carried out using the PAST statistical program to perform principal component analysis (PCA) [17].
Results and Discussion
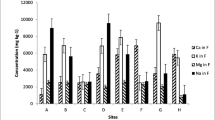
Macro and micro element quantities of cultivated and wild fruits obtained from different locations are depicted in Table 2. The element quantities of fruits showed some changes based on the fruit types. The macroelements detected in the highest amounts in the fruits studied were K, P, Ca, and Mg. While K quantities of the fruits are recorded to be between 5212.77 (“white myrtle”) and 25,550.60 mg/kg (“black nightshade”), P quantities of the fruit samples were characterized to be between 949.08 (“black myrtle”) and 4420.75 mg/kg (“black nightshade”). Calcium and Mg quantities of the fruits were recorded to be between 359.83 (“plum”) and 4330.89 mg/kg (“yellow hawthorn”) to 214.98 (“plum”) and 1852.04 mg/kg (“black nightshade”), respectively. Phosphorus, K, and Mg contents of Black Nightshade wild fruits were found to be much higher than those of cultivated fruits. The most abundant microelement detected in cultivated and wild fruits was B, followed by Fe, Mn, Cu, and Zn (Table 2). Iron and B results of the fruits samples were established to be between 2.69 (“black myrtle”) and 60.13 mg/kg (cherry) to 3.76 (“black myrtle”) and 76.25 mg/kg (“sour cherry”), respectively. While Zn amounts of the fruits vary between 0.84 (“black grape”) and 8.97 mg/kg (“black nightshade”), Mn amounts of the fruits changed to be between 1.83 (“elaeagnus”) and 12.59 mg/kg (“black nightshade”). In addition, Cu values of the fruit samples were assigned to be between 0.94 (“plum”) and 9.40 mg/kg (“black nightshade”). In general, except for “white and black myrtle” fruits, it is thought that other fruits can be good sources of P, K, and Fe. Phosphorus contents of “chery laurel,” “cherry” (“black nightshade”), and “black grape” were partly higher than other fruits. The highest K was detected in black nightshade, “cherry,” “black grape,” “black fig,” and “sour cherry” fruits. The fruits with the highest Ca amount were “hawthorn,” “cherry laurel,” “cherry” and “black myrtle.” While the highest Mg was detected in “black nightshade” and “black fig” fruits, the Mg amounts of other fruits were determined at very low levels. There were significant changes in the Fe content of the fruits, and the lowest Fe was determined in black myrtle, white myrtle, and “elaeagnus” fruits. Zinc contents of “sour cherry,” “cherry,” “black nightshade,” “black fig,” “hawthorn,” “elaeagnus,” and “black myrtle” were higher than those of other fruits. The B absorption ability of cherry laurel, sour cherry, black grape, and cherry was found to be higher than that of other fruits. In general, among the fruits studied, the fruit containing the highest P, K, Mg, Zn, Cu, and Mn was the currant fruit. In previous study, Fe, Cu, Mn, and Zn amounts of the dried apricot fruits changed to be between 10.4 and 80.1, 0.92 and 6.49, 0.97 and 8.27, and 2.96 and 12.0 µg/g, respectively [20]. Cranberry, Lonicera caucasica, “wild plum” and “blackberry” fruits contained 12–53 mg/100 g P, 287–585 mg/100 g K, 3.62–20.24 mg/100 g Na, 4–26.4 mg/100 g Ca, 7.97–12.30 mg/100 g Mg, 0.07–25.38 Fe mg/kg, 0.06–0.29 mg/kg Cu, 0.06–0.6 mg/kg Zn, and 0.05–1.96 mg/kg Mn [4]. In previous study, Dumbrava et al. [6] pointed out that dried brown raisins, dried dates, and dried figs contained 32,175 mg/kg, 6097 mg/kg, and 5730 mg/kg K, respectively. Dumbrava et al. [6] determined 2034 mg/kg Ca, and 1139 mg/kg P in dried figs. Phosphorus and K values of some wild fruits changed between 385 (“blackberry”) and 2538 (fig) to 6114 (medlar) and 18,613 mg/kg (wild strawberry), respectively [1]. Differences in the biogenic element contents of fruit samples may probably be due to the amount of skin content and pulp content of the fruits, and the element contents and climatic conditions of the soil where the fruits grow. Results showed some changes compared to the values of last studies [4, 20]. These changes can be due to fruit skin, fruit pulp, drying type, fruit type, ripening, climatic factors, and soil fertilizer.
Principal component analysis
Fruit varieties (“Black nightshade,” “Sour cherry,” “Cherry,” “Pear,” “Fragrant black grape,” “Persimmon,” “Black fig,” “strawberry tree,” “Hawthorn (Yellow),” “elaeagnus,” “Damask plum,” “Japanese pear,” “Myrtle (White),” and “myrtle (Black)”) and the Pearson correlation (r) between macro (P, K, Ca, and Mg) and micro (Fe, Zn, Mn, Cu, and B) nutrient contents and moisture contents are given in Fig. 2. As can be seen by examining Fig. 2, it has been determined that there are positive relationships between moisture content and nutritional elements of fruit varieties. Although there are positive relationships between P contents of fruits and other nutritional elements, K (r = 0.951**), Mg (r = 0.885**), Zn (r = 0.758**), Cu (r = 0.783**), and Mn ( r = 0.787**) and its contents were reported to have significant and high strength relationships (p < 0.05, r > 0.70). It was determined that there were significant and highly strong positive relationships between the K contents of the varieties and their Mg (r = 0.863**), Zn (r = 0.753**), Cu (r = 0.736**), and Mn (r = 0.732**) contents have been made. While significant and moderately strong positive relationships (p < 0.05, r = 0.30–0.70) were determined between the Mg contents and Cu contents of the fruit varieties (r = 0.627**), Zn (r = 0.729**) and Mn (r = 0.840**) were determined. It has been revealed that there are significant and high-strong positive relationships between their contents and their contents. When looking at the Zn and Cu amounts of the fruits, this study determined that there were significant and moderately strong positive relationships between Zn contents and Cu (r = 0.515**) and Mn (r = 0.594**) contents, and between Cu contents and Mn contents (r = 0.594**).
Fruit varieties (Black berry), sour cherry, cherry, pear, black grape, fragrant black grape, persimmon, black fig, strawberry tree, hawthorn (yellow), oleaster, damask plum, japanese pear, myrtle (white) and murt (black)), the Pearson correlation (r) between macro (P, K, Ca, and Mg) and micro (Fe, Zn, Mn, Cu, and B) nutrient contents and moisture contents
Conclusion
The element detected in the highest amounts in the fruits studied as a macro element was K, followed by P, Ca, and Mg. “Cherry laurel,” “sour cherry,” “cherry,” “pear,” “black nightshade,” “black fig,” and “hawthorn (yellow) fruits contain more K than other fruits. Also, “black fig,” “hawthorn (yellow),” and “myrtle (white)” fruits are richer in Ca than other fruits. The highest P content was detected in “black nightshade” fruits. In addition, “eleagnus” fruit is very rich in Zn. The highest Fe content was detected in “cherry laurel,” “sour cherry,” “cherry,” and “pear” fruits. The most abundant microelement detected in cultivated and wild fruits was B, followed by Fe, Mn, Cu, and Zn in decreasing order. In general, except for “white and black myrtle” fruits, it is thought that other fruits can be good sources of P, K and Fe.
Data Availability
No datasets were generated or analysed during the current study.
References
Al-Juhaimi F, Akçay Kulluk D, Ahmed IAM, Özcan MM, Adiamo O (2023) Quantitative determination of macro and micro elements and heavy metals accumulated in wild fruits analyzed by ICP-OES method. Environ Monit Assess 195:1370
CRA (1999) Analytical Method of the Member Companies of The Corn Refiners Association, Inc, 6th edn. CRA, Washington DC
Demir F, Özcan M (2001) Chemical and technological properties of rose (Rosa canina L.) fruits grown wild in Turkey. J Food Eng 47:333–336
Demir H (2002) Bazı yabani meyve türlerinin besin degerlerinin belirlenmesi üzerinde bir araştırma. Bahçe 31(1–2):33–38
Dhiman P, Soni K, Singh S (2014) Nutritional value of dry fruits and their vital significance - a review. Pharma Tutor 2(3):102–108
Dumbrava DG, Bordean DM, Moldovan C, Popa VM, Raba DN, Barbecea A, Cocan I, Alexa EC (2020) Content of total polyphenols, vitamin C, mineral elements and the antioxidant activity of some dried fruits from the Romanian market. J Agroaliment Process Technol 26:325–329
Frossard E, Bucher M, Mächler F, Mozafar A, Hurrell R (2000) Potential for increasing the content and bioavailability of Fe, Zn and Ca in plants for human nutrition. J Sci Food Agric 80:861–879
Gall JE, Boyd RS, Rajakaruna N (2015) Transfer of heavy metals through terrestrial food webs: A review. Environ Monit Assess 187:201
İmer Y (2016) Çeşitli soğuk pres yağların bazı mikro ve makro element içeriklerinin belirlenmesi. Yüksek Lisans Tezi, Namık Kemal Üniversitesi, Fen Bilimleri Enstitüsü, Tekirdağ, Turkey
Lechaudel M, Joas J, Caro Y, Genard M, Jannoyer M (2005) Leaf:fruit ratio and irrigation supply affect seasonal changes in minerals, organic acids and sugars of mango fruit. J Sci Food Agric 85:251–260
Momen AA, Zachariadis GA, Anthemidis AN, Stratis JA (2007) Use of fractional factorial design for optimization of digestion procedures followed by multi-element determination of essential and non-essential elements in nuts using ICP-OES technique. Talanta 71:443–451. https://doi.org/10.1016/j.talanta.2006.04.018
Moodley R, Kindness A, Jonnalagadda SB (2007) Elemental composition and chemical characteristics of five edible nuts (almond, Brazil, pecan, macadamia and walnut) consumed in Southern Africa. J Environ Sci Health B 42(5):585–591. https://doi.org/10.1080/03601230701391591
Nahar N, Rahaman S, Mosiihuzzaman (1990) Analysis of carbohydrates in seven edible fruits of Bangladesh. J Sci Food Agric 5:185–192
Naozuka J, Marana SR, Oliveira PV (2010) Water-soluble Cu, Fe, Mn and Zn species in nuts and seeds. J Food Compos Anal 23:78–85
Özcan MM, Uslu N (2023) The effect of thermal treatment on antioxidant activity and changes in bioactive and phenolic compounds of three pear (Pyrus spp.) varieties slices. Food Humanity 1:281–288
Özcan MM, AkçayKulluk D, Gökmen Yılmaz F, Dursun N (2023) Macro-, micro-, and heavy metal element levels in different parts of celery (Apium graveolens L.) plant. Environ Monit Assess 1195(5):550
Pulliainen TK, Wallin HC (1996) Determination of total phosphorus in foods by colorimetry: Summary of NMKL. J AOAC Int 79(6):1408–1410
Rodushkin I, Engstrom E, Sorlin D, Baxter D (2008) Levels of inorganic constituents in raw nuts and seeds on the Swedish market. Sci Total Environ 392:290–304. https://doi.org/10.1016/j.scitotenv.2007.11.024
San B, Yildirim AN, Pola TM, Yildirim F (2009) Mineral composition of leaves and fruits of some promising jujube (Zizyphus jujuba Miller) genotypes. Asian J Chem 21(4):2898–2902
Saracoglu S, Tuzen M, Soylak M (2009) Evaluation of trace element contents of dried apricot samples from Turkey. J Hazard Mater 167:647–652. https://doi.org/10.1016/j.jhazmat.2009.01.011
Suliburska J, Krejpcio Z (2014) Evaluation of the content and bioaccessibility of iron, zinc, calcium and magnesium from groats, rice, leguminous grains and nuts. J Food Sci Technol 51:589–594. https://doi.org/10.1007/s13197-011-0535-5
Savaşlı E, Önder O, Karaduman Y, Dayıoğlu R, Özen D, Özdemir S, Akın A, Tunca ZS, Demir B, Aydın N (2019) The effect of soil and foliar ürea application at heading stage on grain yield and quality traits of bread wheat (Triticium aestivum L.). Turk J Agric Sci Technol 7:1928–1936
Şahan Y, Başoğlu F (2003) Ağır metal iyonlarının insan sağlığına etkisi. Dünya Gıda 8(3):70–76
Tošic SB, Mitic SS, Velimirovic DS, Stojanovic GS, Pavlovic AN, Pecev-Marinkovic ET (2015) Elemental composition of edible nuts: fast optimization and validation procedure of an ICP- OES method. J Sci Food Agric 95:2271–2278
Türközü D, Şanlıer N (2014) Gıdalardaki ağır metal kontaminasyonları: Bulaşma kaynakları, sağlık riskleri ve ulusal/uluslararası standartlar. Gıda Teknolojisi Elektronik Dergisi 9(3):29–46
Wuilloud RG, Kannamkumarath SS, Caruso JA (2004) Speciation of nickel, copper, zinc, and manganese in different edible nuts: a comparative study of molecular size distribution by SEC–UV–ICP–MS. Anal Bioanal Chem 379:495–503. https://doi.org/10.1007/s00216-004-2592-3
Acknowledgements
The authors extend their appreciation to Researchers Supporting Project Number (RSP2024R083), King Saud University, Riyadh, Saudi Arabia.
Funding
Open access funding provided by the Scientific and Technological Research Council of Türkiye (TÜBİTAK).
Author information
Authors and Affiliations
Contributions
Fahad AlJuhaimi; methodology, editing; Duygu Akçay Kulluk: formal analysis, Isam Ali Mohamed Ahmed: validation, statistical analysis, editing; formal analysis; Mehmet Musa Özcan: Supervision, methodology, formal analysis, writing-reviewing; Emad Karrar: editing; investifation, software, validation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
AlJuhaimi, F., Kulluk, D.A., Ahmed, I.A.M. et al. Investigation of accumulation of element contents in some wild and cultivated dried fruits. Biol Trace Elem Res (2024). https://doi.org/10.1007/s12011-024-04165-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12011-024-04165-w