Abstract
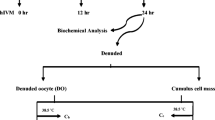
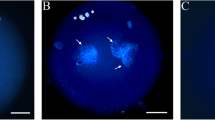
Selenium (Se), an essential trace element, plays an important role in the antioxidative defense mechanism, and it has been proven to improve fertility and reproductive efficiency in dairy cattle. The present study evaluated the potential protective action of Se supplement of in vitro maturation (IVM) media on the maturation and subsequent development of bovine cumulus-oocyte complexes (COCs) exposed to heat stress (HS). The treatment with Se improved the viability of cumulus cells (CCs) and oocytes (P < 0.05). The proportion of oocytes reached metaphase II (MII) and those arrested at metaphase I (MI) was greater and lower in treatment than control respectively (P < 0.05). Supplementation with Se increased the percentage of cleaved embryos, total blastocysts, and blastocyst/cleavage ratio (P < 0.05). Moreover, the upregulation of CCND1, SEPP1, GPX-4, SOD, CAT, and downregulation of GRP78, CHOP, and BAX in both Se-treated CCs and oocytes were recorded. The upregulation of NRF2 was detected in Se-treated CCs other than in oocytes, which showed upregulation of IGF2R and SOX-2 as the markers of quality as well. Se supplement in IVM media improved the viability, maturation, and the level of transcripts related to antioxidant defense and quality of heat-treated oocytes, which coincided with greater subsequent development outcomes. Se ameliorated the viability of CCs along with upregulation of antioxidative candidate gene expression and downregulation of apoptosis-related ones to support their protective role on restoring the quality of oocytes against compromising effects of HS.




Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Belhadj Slimen I, Najar T, Ghram A, Abdrrabba M (2016) Heat stress effects on livestock: molecular, cellular and metabolic aspects, a review. J Anim Physiol Anim Nutr 100:401–412. https://doi.org/10.1111/jpn.12379
Akbarinejad V, Gharagozlou F, Vojgani M (2017) Temporal effect of maternal heat stress during gestation on the fertility and anti-Müllerian hormone concentration of offspring in bovine. Theriogenology 99:69–78. https://doi.org/10.1016/j.theriogenology.2017.05.018
Lord-Fontaine S, Averill-Bates DA (2002) Heat shock inactivates cellular antioxidant defenses against hydrogen peroxide: protection by glucose. Free Radic Biol Med 32:752–765. https://doi.org/10.1016/S0891-5849(02)00769-4
Wolfenson D, Roth Z, Meidan R (2000) Impaired reproduction in heat-stressed cattle: basic and applied aspects. Anim Reprod Sci 60:535–547. https://doi.org/10.1016/S0378-4320(00)00102-0
Payton RR, Rispoli LA, Nagle KA, Gondro C, Saxton AM, Voy BH et al (2018) Mitochondrial-related consequences of heat stress exposure during bovine oocyte maturation persist in early embryo development. J Reprod Dev 64:243–251. https://doi.org/10.1262/jrd.2017-160
Aroyo A, Yavin S, Arav A, Roth Z (2007) Maternal hyperthermia disrupts developmental competence of follicle-enclosed oocytes: in vivo and ex vivo studies in mice. Theriogenology 67:1013–1021. https://doi.org/10.1016/j.theriogenology.2006.12.001
Roth Z, Hansen P (2004) Involvement of apoptosis in disruption of developmental competence of bovine oocytes by heat shock during maturation. Biol Reprod 71:1898–1906. https://doi.org/10.1095/biolreprod.104.031690
Wang J-Z, Sui H-S, Miao D-Q, Liu N, Zhou P, Ge L et al (2009) Effects of heat stress during in vitro maturation on cytoplasmic versus nuclear components of mouse oocytes. Reproduction 137:181
Ferreira RM, Chiaratti MR, Macabelli CH, Rodrigues CA, Ferraz ML, Watanabe YF et al (2016) The infertility of repeat-breeder cows during summer is associated with decreased mitochondrial DNA and increased expression of mitochondrial and apoptotic genes in oocytes. Biol Reprod 94(66):1–10
Hale BJ, Hager CL, Seibert JT, Selsby JT, Baumgard LH, Keating AF et al (2017) Heat stress induces autophagy in pig ovaries during follicular development. Biol Reprod 97:426–437. https://doi.org/10.1093/biolre/iox097
Naseer Z, Ahmad E, Epikmen ET, Uçan U, Boyacioğlu M, İpek E et al (2017) Quercetin supplemented diet improves follicular development, oocyte quality, and reduces ovarian apoptosis in rabbits during summer heat stress. Theriogenology 96:136–141. https://doi.org/10.1016/j.theriogenology.2017.03.029
Shaeib F, Khan SN, Ali I, Thakur M, Saed G, Dai J et al (2016) The defensive role of cumulus cells against reactive oxygen species insult in metaphase II mouse oocytes. Reprod Sci 23:498–507
Mehdi Y, Hornick J-L, Istasse L, Dufrasne I (2013) Selenium in the environment, metabolism and involvement in body functions. Molecules 18:3292–3311. https://doi.org/10.3390/molecules18033292
Mehdi Y, Dufrasne I (2016) Selenium in cattle: a review. Molecules 21:545. https://doi.org/10.3390/molecules21040545
Kommisrud E, Østerås O, Vatn T (2005) Blood selenium associated with health and fertility in Norwegian dairy herds. Acta Vet Scand 46:1–12. https://doi.org/10.1186/1751-0147-46-229
Brigelius-Flohé R, Maiorino M (2013) Glutathione peroxidases. Biochim Biophys Acta Gen Subj 1830:3289–3303. https://doi.org/10.1016/j.bbagen.2012.11.020
Córdova B, Morató R, Izquierdo D, Paramio T, Mogas T (2010) Effect of the addition of insulin-transferrin-selenium and/or L-ascorbic acid to the in vitro maturation of prepubertal bovine oocytes on cytoplasmic maturation and embryo development. Theriogenology 74:1341–1348. https://doi.org/10.1016/j.theriogenology.2010.06.003
Lizarraga RM, Anchordoquy JM, Galarza EM, Farnetano NA, Carranza-Martin A, Furnus CC et al (2020) Sodium selenite improves in vitro maturation of bos primigenius taurus oocytes. Biol Trace Elem Res 197:149–158. https://doi.org/10.1007/s12011-019-01966-2
Xiong X, Lan D, Li J, Lin Y, Li M (2018) Selenium supplementation during in vitro maturation enhances meiosis and developmental capacity of yak oocytes. Anim Sci J 89:298–306. https://doi.org/10.1111/asj.12894
Uhm SJ, Gupta MK, Yang JH, Lee SH, Lee HT (2007) Selenium improves the developmental ability and reduces the apoptosis in porcine parthenotes. Mol Reprod Dev Gamete Res 74:1386–1394. https://doi.org/10.1002/mrd.20701
Makki M, Saboori E, Sabbaghi MA, Aram R, Fallahian MH, Peyghambari F et al (2012) Effects of selenium, calcium and calcium ionophore on human oocytes in vitro maturation in a chemically defined medium. Iran J Reprod Med 10:343–348
Tareq K, Akter QS, Khandoker MAMY, Tsujii H (2012) Selenium and vitamin E improve the in vitro maturation, fertilization and culture to blastocyst of porcine oocytes. J Reprod Dev 58:621–628. https://doi.org/10.1262/jrd.2012-064
Tervit H, Whittingham D, Rowson L (1972) Successful culture in vitro of sheep and cattle ova. Reproduction 30:493–497
Davoodian N, Kadivar A, Davoodian N, Ahmadi E, Nazari H, Mehrban H (2022) The effect of quercetin in the maturation media on cumulus-granulosa cells and the developmental competence of bovine oocytes. Theriogenology 189:262–269. https://doi.org/10.1016/j.theriogenology.2022.06.026
Pfaffl MW, Horgan GW, Dempfle L (2002) Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res 30:e36–e36. https://doi.org/10.1093/nar/30.9.e36
Ruijter J, Ramakers C, Hoogaars W, Karlen Y, Bakker O, Van den Hoff M et al (2009) Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res 37:e45–e45. https://doi.org/10.1093/nar/gkp045
Payton RR, Romar R, Coy P, Saxton AM, Lawrence JL, Edwards JL (2004) Susceptibility of bovine germinal vesicle-stage oocytes from antral follicles to direct effects of heat stress in vitro. Biol Reprod 71:1303–1308. https://doi.org/10.1095/biolreprod.104.029892
Edwards J, Saxton A, Lawrence J, Payton R, Dunlap J (2005) Exposure to a physiologically relevant elevated temperature hastens in vitro maturation in bovine oocytes. J Dairy Sci 88:4326–4333. https://doi.org/10.3168/jds.S0022-0302(05)73119-2
Báez F, Camargo Á, Reyes AL, Márquez A, Paula-Lopes F, Viñoles C (2019) Time-dependent effects of heat shock on the zona pellucida ultrastructure and in vitro developmental competence of bovine oocytes. Reprod Biol 19:195–203. https://doi.org/10.1016/j.repbio.2019.06.002
Residiwati G, Azari-Dolatabad N, Tuska HSA, Sidi S, Van Damme P, Benedetti C et al (2021) Effect of lycopene supplementation to bovine oocytes exposed to heat shock during in vitro maturation. Theriogenology 173:48–55. https://doi.org/10.1016/j.theriogenology.2021.07.014
Zou Y, Shao J, Li Y, Zhao F-Q, Liu J-X, Liu H (2019) Protective effects of inorganic and organic selenium on heat stress in bovine mammary epithelial cells. Oxid Med Cell Longev 2019:1503478. https://doi.org/10.1155/2019/1503478
Chermuła B, Brązert M, Jeseta M, Ożegowska K, Sujka-Kordowska P, Konwerska A et al (2018) The unique mechanisms of cellular proliferation, migration and apoptosis are regulated through oocyte maturational development—a complete transcriptomic and histochemical study. Int J Mol Sci 20:84. https://doi.org/10.3390/ijms20010084
Ge L, Han D, Lan GC, Zhou P, Liu Y, Zhang X et al (2008) Factors affecting the in vitro action of cumulus cells on the maturing mouse oocytes. Mol Reprod Dev Gamete Res 75:136–142. https://doi.org/10.1002/mrd.20753
Feuerstein P, Cadoret V, Dalbies-Tran R, Guerif F, Bidault R, Royère D (2007) Gene expression in human cumulus cells: one approach to oocyte competence. Hum Reprod 22:3069–3077. https://doi.org/10.1093/humrep/dem336
Tesfaye D, Ghanem N, Carter F, Fair T, Sirard M-A, Hoelker M et al (2009) Gene expression profile of cumulus cells derived from cumulus–oocyte complexes matured either in vivo or in vitro. Reprod Fertil Dev 21:451–461. https://doi.org/10.1071/RD08190
Ferreira E, Vireque A, Adona PR, Meirelles FV, Ferriani RA, Navarro PAAS (2009) Cytoplasmic maturation of bovine oocytes: structural and biochemical modifications and acquisition of developmental competence. Theriogenology 71:836–848. https://doi.org/10.1016/j.theriogenology.2008.10.023
Watson A (2007) Oocyte cytoplasmic maturation: a key mediator of oocyte and embryo developmental competence. J Anim Sci 85:E1–E3. https://doi.org/10.2527/jas.2006-432
Gilchrist RB, Thompson JG (2007) Oocyte maturation: emerging concepts and technologies to improve developmental potential in vitro. Theriogenology 67:6–15. https://doi.org/10.1016/j.theriogenology.2006.09.027
Takahashi M (2012) Oxidative stress and redox regulation on in vitro development of mammalian embryos. J Reprod Dev 58:1–9. https://doi.org/10.1262/jrd.11-138N
Sunde RA (1990) Molecular biology of selenoproteins. Annu Rev Nutr 10:451–474. https://doi.org/10.1146/annurev.nu.10.070190.002315
Luberda Z (2005) The role of glutathione in mammalian gametes. Reprod Biol 5:5–17
Feugang J-M, De Roover R, Moens A, Léonard S, Dessy F, Donnay I (2004) Addition of β-mercaptoethanol or Trolox® at the morula/blastocyst stage improves the quality of bovine blastocysts and prevents induction of apoptosis and degeneration by prooxidant agents. Theriogenology 61:71–90. https://doi.org/10.1016/S0093-691X(03)00191-2
Ansar S, Alshehri SM, Abudawood M, Hamed SS, Ahamad T (2017) Antioxidant and hepatoprotective role of selenium against silver nanoparticles. Int J Nanomedicine 12:7789. https://doi.org/10.2147/IJN.S136748
Abedelahi A, Salehnia M, Allameh A, Davoodi D (2010) Sodium selenite improves the in vitro follicular development by reducing the reactive oxygen species level and increasing the total antioxidant capacity and glutathione peroxide activity. Hum Reprod 25:977–985. https://doi.org/10.1093/humrep/deq002
Basini G, Tamanini C (2000) Selenium stimulates estradiol production in bovine granulosa cells: possible involvement of nitric oxide☆. Domest Anim Endocrinol 18:1–17. https://doi.org/10.1016/S0739-7240(99)00059-4
Melo E, Cordeiro D, Pellegrino R, Wei Z, Daye Z, Nishimura R et al (2017) Identification of molecular markers for oocyte competence in bovine cumulus cells. Anim Genet 48:19–29. https://doi.org/10.1111/age.12496
Assidi M, Dieleman SJ, Sirard M-A (2010) Cumulus cell gene expression following the LH surge in bovine preovulatory follicles: potential early markers of oocyte competence. Reproduction 140:835. https://doi.org/10.1530/REP-10-0248
Rakha SI, Elmetwally MA, El-Sheikh Ali H, Balboula A, Mahmoud AM, Zaabel SM (2022) Importance of Antioxidant Supplementation during In Vitro Maturation of Mammalian Oocytes. Vet Sci 9:439. https://doi.org/10.3390/vetsci9080439
Ispada J, Rodrigues T, Risolia PHB, Lima R, Gonçalves D, Rettori D et al (2018) Astaxanthin counteracts the effects of heat shock on the maturation of bovine oocytes. Reprod Fertil Dev 30:1169–1179. https://doi.org/10.1071/RD17271
Eshtiyaghi M, Deldar H, Pirsaraei ZA, Shohreh B (2016) Royal jelly may improve the metabolism of glucose and redox state of ovine oocytes matured in vitro and embryonic development following in vitro fertilization. Theriogenology 86:2210–2221. https://doi.org/10.1016/j.theriogenology.2016.07.019
Xiong X, Lan D, Li J, Yin S, Xiong Y, Zi X (2019) Identification of differential abundances of mRNA transcript in cumulus cells and CCND1 associated with yak oocyte developmental competence. Anim Reprod Sci 208:106135. https://doi.org/10.1016/j.anireprosci.2019.106135
Anguita B, Paramio M-T, Jiménez-Macedo AR, Morató R, Mogas T, Izquierdo D (2008) Total RNA and protein content, Cyclin B1 expression and developmental competence of prepubertal goat oocytes. Anim Reprod Sci 103:290–303. https://doi.org/10.1016/j.anireprosci.2006.12.018
Chakravarthi VP, Kona S, Kumar AS, Bhaskara M, Rao V (2016) Stage specific expression of cell cycle genes during in vivo or in vitro development of ovarian follicles in sheep. Small Rumin Res 143:1–7. https://doi.org/10.1016/j.smallrumres.2016.08.011
Kamada H, Ikumo H (1997) Effect of selenium on cultured bovine luteal cells. Anim Reprod Sci 46:203–211. https://doi.org/10.1016/S0378-4320(96)01617-X
Gladyshev VN, Arnér ES, Berry MJ, Brigelius-Flohé R, Bruford EA, Burk RF et al (2016) Selenoprotein gene nomenclature. J Biol Chem 291:24036–24040. https://doi.org/10.1074/jbc.M116.756155
Papp LV, Lu J, Holmgren A, Khanna KK (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9:775–806. https://doi.org/10.1089/ars.2007.1528
Chan JM, Darke AK, Penney KL, Tangen CM, Goodman PJ, Lee G-SM et al (2016) Selenium-or vitamin E–related gene variants, interaction with supplementation, and risk of high-grade prostate cancer in SELECT. Cancer Epidemiol Biomarkers Prev 25:1050–1058. https://doi.org/10.1158/1055-9965.EPI-16-0104
Qazi IH, Angel C, Yang H, Pan B, Zoidis E, Zeng C-J et al (2018) Selenium, selenoproteins, and female reproduction: a review. Molecules 23:3053. https://doi.org/10.3390/molecules23123053
Brigelius-Flohé R, Flohé L (2020) Regulatory phenomena in the glutathione peroxidase superfamily. Antioxid Redox Signal 33:498–516. https://doi.org/10.1089/ars.2019.7905
Imai H, Nakagawa Y (2003) Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radic Biol Med 34:145–169. https://doi.org/10.1016/S0891-5849(02)01197-8
Hosnedlova B, Kepinska M, Skalickova S, Fernandez C, Ruttkay-Nedecky B, Malevu TD et al (2017) A summary of new findings on the biological effects of selenium in selected animal species—a critical review. Int J Mol Sci 18:2209. https://doi.org/10.3390/ijms18102209
Ceko MJ, O’Leary S, Harris HH, Hummitzsch K, Rodgers RJ (2016) Trace elements in ovaries: measurement and physiology. Biol Reprod 94(86):1–14. https://doi.org/10.1095/biolreprod.115.137240
Demirci K, Nazıroğlu M, Övey İS, Balaban H (2017) Selenium attenuates apoptosis, inflammation and oxidative stress in the blood and brain of aged rats with scopolamine-induced dementia. Metab Brain Dis 32:321–329. https://doi.org/10.1007/s11011-016-9903-1
Fang J, Zheng Z, Yang Z, Peng X, Zuo Z, Cui H et al (2018) Ameliorative effects of selenium on the excess apoptosis of the jejunum caused by AFB1 through death receptor and endoplasmic reticulum pathways. Toxicol Res 7:1108–1119. https://doi.org/10.1039/c8tx00068a
Khera A, Vanderlelie JJ, Holland O, Perkins AV (2017) Overexpression of endogenous anti-oxidants with selenium supplementation protects trophoblast cells from reactive oxygen species-induced apoptosis in a Bcl-2-dependent manner. Biol Trace Elem Res 177:394–403. https://doi.org/10.1007/s12011-016-0870-5
Kensler TW, Wakabayashi N, Biswal S (2007) Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol 47:89–116. https://doi.org/10.1146/annurev.pharmtox.46.120604.141046
Hayes JD, Dinkova-Kostova AT (2014) The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem Sci 39:199–218. https://doi.org/10.1016/j.tibs.2014.02.002
Kwak M-K, Wakabayashi N, Greenlaw JL, Yamamoto M, Kensler TW (2003) Antioxidants enhance mammalian proteasome expression through the Keap1-Nrf2 signaling pathway. Mol Cell Biol 23:8786–8794. https://doi.org/10.1128/MCB.23.23.8786-8794.2003
McMahon M, Itoh K, Yamamoto M, Hayes JD (2003) Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem 278:21592–21600. https://doi.org/10.1074/jbc.M300931200
Wang M, Li Y, Molenaar A, Li Q, Cao Y, Shen Y et al (2021) Vitamin E and selenium supplementation synergistically alleviate the injury induced by hydrogen peroxide in bovine granulosa cells. Theriogenology 170:91–106. https://doi.org/10.1016/j.theriogenology.2021.04.015
Guan F, Zhang S, Fan L, Sun Y, Ma Y, Cao C et al (2023) Kunling Wan improves oocyte quality by regulating the PKC/Keap1/Nrf2 pathway to inhibit oxidative damage caused by repeated controlled ovarian hyperstimulation. J Ethnopharmacol 301:115777. https://doi.org/10.1016/j.jep.2022.115777
Huang Z, Pang Y, Hao H, Du W, Zhao X, Zhu H (2018) Effects of epigallocatechin-3-gallate on bovine oocytes matured in vitro. Asian-Australas J Anim Sci 31:1420. https://doi.org/10.5713/ajas.17.0880
Khatun H, Wada Y, Konno T, Tatemoto H, Yamanaka K-i (2020) Endoplasmic reticulum stress attenuation promotes bovine oocyte maturation in vitro. Reproduction 159:361–370. https://doi.org/10.1530/REP-19-0492
Wu LL, Russell DL, Norman RJ, Robker RL (2012) Endoplasmic reticulum (ER) stress in cumulus-oocyte complexes impairs pentraxin-3 secretion, mitochondrial membrane potential (ΔΨm), and embryo development. Mol Endocrinol 26:562–573. https://doi.org/10.1210/me.2011-1362
Iurlaro R, Muñoz-Pinedo C (2016) Cell death induced by endoplasmic reticulum stress. FEBS J 283:2640–2652. https://doi.org/10.1111/febs.13598
Walter P, Ron D (2011) The unfolded protein response: from stress pathway to homeostatic regulation. Science 334:1081–1086. https://doi.org/10.1126/science.1209038
Alemu TW, Pandey HO, Wondim DS, Gebremedhn S, Neuhof C, Tholen E et al (2018) Oxidative and endoplasmic reticulum stress defense mechanisms of bovine granulosa cells exposed to heat stress. Theriogenology 110:130–141. https://doi.org/10.1016/j.theriogenology.2017.12.042
Lin P, Yang Y, Li X, Chen F, Cui C, Hu L et al (2012) Endoplasmic reticulum stress is involved in granulosa cell apoptosis during follicular atresia in goat ovaries. Mol Rerod Dev 79:423–432. https://doi.org/10.1002/mrd.22045
Spears JW, Weiss WP (2008) Role of antioxidants and trace elements in health and immunity of transition dairy cows. Vet J 176:70–76. https://doi.org/10.1016/j.tvjl.2007.12.015
Xiong Y, Yin Q, Jin E, Chen H, He S (2020) Selenium attenuates chronic heat stress-induced apoptosis via the inhibition of endoplasmic reticulum stress in mouse granulosa cells. Molecules 25:557. https://doi.org/10.3390/molecules25030557
Abazarikia AH, Zhandi M, Shakeri M, Towhidi A, Yousefi AR (2020) In vitro supplementation of trans-10, cis-12 conjugated linoleic acid ameliorated deleterious effect of heat stress on bovine oocyte developmental competence. Theriogenology 142:296–302. https://doi.org/10.1016/j.theriogenology.2019.10.028
Wang M, Wey S, Zhang Y, Ye R, Lee AS (2009) Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid Redox Signal 11:2307–2316. https://doi.org/10.1089/ars.2009.2485
Kadowaki H, Nishitoh H (2013) Signaling pathways from the endoplasmic reticulum and their roles in disease. Genes 4:306–333. https://doi.org/10.3390/genes4030306
Rasheva VI, Domingos PM (2009) Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis 14:996–1007. https://doi.org/10.1007/s10495-009-0341-y
Wang L, Feng H, Ma YZ, Cang M, Li H, Yan Z et al (2009) Expression of IGF receptors and its ligands in bovine oocytes and preimplantation embryos. Anim Reprod Sci 114:99–108. https://doi.org/10.1016/j.anireprosci.2008.09.019
Yaseen M, Wrenzycki C, Herrmann D, Carnwath J, Niemann H (2001) Changes in the relative abundance of mRNA transcripts for insulin-like growth factor (IGF-I and IGF-II) ligands and their receptors (IGF-IR/IGF-IIR) in preimplantation bovine embryos derived from different in vitro systems. Reproduction 122:601–610. https://doi.org/10.1530/rep.0.1220601
Gendelman M, Aroyo A, Yavin S, Roth Z (2010) Seasonal effects on gene expression, cleavage timing, and developmental competence of bovine preimplantation embryos. Reproduction 140:73–82. https://doi.org/10.1530/REP-10-0055
Funding
This work was supported by Shahrekord University, Shahrekord, Iran (grant numbers: 0GRD34M40481).
Author information
Authors and Affiliations
Contributions
Najmeh Davoodian, Mehran Arabi, and Ali Kadivar contributed to the studies conception and design. The manuscript writing was conducted by Najmeh Davoodian. Material preparation and data collection were performed by Najmeh Davoodian and Shervin Toosinia. The molecular analysis and statistical analysis were carried out by Ali Kadivar. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics Approval
No approval of research ethics committees was required to accomplish the goals of this study because all the experiments were conducted with materials collected from a local slaughterhouse.
Consent to Participate
All the authors contributed to the performance of the study. The final manuscript was read and approved by all the authors.
Consent for Publication
All the authors gave their consent for the publication of the research.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Toosinia, S., Davoodian, N., Arabi, M. et al. Ameliorating Effect of Sodium Selenite on Developmental and Molecular Response of Bovine Cumulus-Oocyte Complexes Matured in Vitro Under Heat Stress Condition. Biol Trace Elem Res 202, 161–174 (2024). https://doi.org/10.1007/s12011-023-03678-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03678-0