Abstract
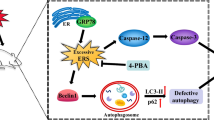
Fluoride can cause developmental neurotoxicity; however, the precise mechanism has yet to be determined. We aimed to explore the possible role and mechanism of fluoride-induced developmental neurotoxicity, specifically the significance of the lysosomal stress response. As an in vivo model, Sprague Dawley rats were exposed to sodium fluoride (NaF) from embryo to 2 months of age. We found that NaF caused autophagic flux blockage and apoptosis in the rat hippocampus. These results were validated in human neuroblastoma (SH-SY5Y) cells in vitro. In addition, in SH-SY5Y cells, NaF hindered autophagosome-lysosome fusion, decreased lysosomal degradation, and elevated lysosomal pH, which is the most prominent hallmark of a lysosomal stress response. Interestingly, rapamycin promoted autophagosome-lysosome fusion, effectively restoring autophagic flux and reducing apoptosis. Notably, bafilomycin A1, a lysosomal lumen alkalizer, unsurprisingly exacerbated the NaF-induced increase in lysosomal pH and decreased lysosomal degradability, as well as enhanced apoptosis of SH-SY5Y cells. In conclusion, our results suggest that NaF exposure initiates excessive lysosomal stress response, resulting in elevated lysosomal pH, decreased lysosomal degradation, and blocked autophagic flux, which leads to neuronal apoptosis. Thus, the lysosomal stress response may be a promising target for the prevention and treatment of fluoride-induced developmental neurotoxicity.
Graphical Abstract

Highlights
-
1. NaF caused autophagic flux blockage and apoptosis in vivo and in vitro
-
2. NaF hindered autophagosome-lysosome fusion and elevated lysosomal pH
-
3. NaF caused excessive lysosomal stress response and impaired lysosomal degradation





Similar content being viewed by others
References
Zhang Y, Wu J, Jiang L, Lu C, Huang Z, Liu B (2021) Prospects for the role of ferroptosis in fluorosis[J]. Front Physiol 12:773055. https://doi.org/10.3389/fphys.2021.773055
Onipe T, Edokpayi JN, Odiyo JO (2020) A review on the potential sources and health implications of fluoride in groundwater of Sub-Saharan Africa[J]. J Environ Sci Health A Tox Hazard Subst Environ Eng 55(9):1078–1093. https://doi.org/10.1080/10934529.2020.1770516
Solanki YS, Agarwal M, Gupta AB, Gupta S, Shukla P (2022) Fluoride occurrences, health problems, detection, and remediation methods for drinking water: a comprehensive review[J]. Sci Total Environ 807(Pt 1):150601. https://doi.org/10.1016/j.scitotenv.2021.150601
Su H, Kang W, Kang N, Liu J, Li Z (2021) Hydrogeochemistry and health hazards of fluoride-enriched groundwater in the Tarim Basin, China[J]. Environ Res 200:111476. https://doi.org/10.1016/j.envres.2021.111476
Cárdenas-González M, Jacobo Estrada T, Rodríguez-Muñoz R, Barrera-Chimal J, Bobadilla NA, Barbier OC, Del Razo LM (2016) Sub-chronic exposure to fluoride impacts the response to a subsequent nephrotoxic treatment with gentamicin[J]. J Appl Toxicol 36(2):309–319. https://doi.org/10.1002/jat.3186
Chaithra B, Sarjan HN, Shivabasavaiah (2020) A comparative analysis of fluoride-contaminated groundwater and sodium fluoride-induced reproductive toxicity and its reversibility in male rats[J]. Biol Trace Elem Res 197(2):507–521
Wang D, Cao L, Zhou X, Wang G, Ma Y, Hao X, Fan H (2022) Mitigation of honokiol on fluoride-induced mitochondrial oxidative stress, mitochondrial dysfunction, and cognitive deficits through activating AMPK/PGC-1α/Sirt3[J]. J Hazard Mater 437:129381. https://doi.org/10.1016/j.jhazmat.2022.129381
Castiblanco-Rubio GA, Martinez-Mier EA (2022) Fluoride metabolism in pregnant women: a narrative review of the literature[J]. Metabolites 12(4):324. https://doi.org/10.3390/metabo12040324
Łukomska A, Baranowska-Bosiacka I, Dec K, Pilutin A, Tarnowski M, Jakubczyk K, Żwierełło W, Skórka-Majewicz M, Chlubek D, Gutowska I (2020) Changes in gene and protein expression of metalloproteinase-2 and -9 and their inhibitors TIMP2 and TIMP3 in different parts of fluoride-exposed rat brain[J]. Int J Mol Sci 22(1):391. https://doi.org/10.3390/ijms22010391
O’mullane DM, Baez RJ, Jones S, Lennon MA, Petersen PE, Rugg-Gunn AJ, Whelton H, Whitford GM (2016) Fluoride and oral health[J]. Community Dent Health 33(2):69–99. https://doi.org/10.1922/CDH_3707O’Mullane31
Gundacker C, Forsthuber M, Szigeti T, Kakucs R, Mustieles V, Fernandez MF, Bengtsen E, Vogel U, Hougaard KS, Saber AT (2021) Lead (Pb) and neurodevelopment: a review on exposure and biomarkers of effect (BDNF, HDL) and susceptibility[J]. Int J Hyg Environ Health 238:113855. https://doi.org/10.1016/j.ijheh.2021.113855
Heyer DB, Meredith RM (2017) Environmental toxicology: sensitive periods of development and neurodevelopmental disorders[J]. Neurotoxicology 58:23–41. https://doi.org/10.1016/j.neuro.2016.10.017
Hollander JA, Cory-Slechta DA, Jacka FN, Szabo ST, Guilarte TR, Bilbo SD, Mattingly CJ, Moy SS, Haroon E, Hornig M, Levin ED, Pletnikov MV, Zehr JL, Mcallister KA, Dzierlenga AL, Garton AE, Lawler CP, Ladd-Acosta C (2020) Beyond the looking glass: recent advances in understanding the impact of environmental exposures on neuropsychiatric disease[J]. Neuropsychopharmacology 45(7):1086–1096. https://doi.org/10.1038/s41386-020-0648-5
Huang Y, Dai Y, Li M, Guo L, Cao C, Huang Y, Ma R, Qiu S, Su X, Zhong K, Huang Y, Gao H, Bu Q (2021) Exposure to cadmium induces neuroinflammation and impairs ciliogenesis in hESC-derived 3D cerebral organoids[J]. Sci Total Environ 797:149043. https://doi.org/10.1016/j.scitotenv.2021.149043
Bashash M, Thomas D, Hu H, Martinez-Mier EA, Sanchez BN, Basu N, Peterson KE, Ettinger AS, Wright R, Zhang Z, Liu Y, Schnaas L, Mercado-García A, Téllez-Rojo MM, Hernández-Avila M (2017) Prenatal fluoride exposure and cognitive outcomes in children at 4 and 6–12 years of age in Mexico[J]. Environ Health Perspect 125(9):097017. https://doi.org/10.1289/ehp655
Green R, Lanphear B, Hornung R, Flora D, Martinez-Mier EA, Neufeld R, Ayotte P, Muckle G, Till C (2019) Association between maternal fluoride exposure during pregnancy and IQ scores in offspring in Canada[J]. JAMA Pediatr 173(10):940–948. https://doi.org/10.1001/jamapediatrics.2019.1729
Bashash M, Marchand M, Hu H, Till C, Martinez-Mier EA, Sanchez BN, Basu N, Peterson KE, Green R, Schnaas L, Mercado-García A, Hernández-Avila M, Téllez-Rojo MM (2018) Prenatal fluoride exposure and attention deficit hyperactivity disorder (ADHD) symptoms in children at 6–12 years of age in Mexico City[J]. Environ Int 121(Pt 1):658–666. https://doi.org/10.1016/j.envint.2018.09.017
Cui Y, Zhang B, Ma J, Wang Y, Zhao L, Hou C, Yu J, Zhao Y, Zhang Z, Nie J, Gao T, Zhou G, Liu H (2018) Dopamine receptor D2 gene polymorphism, urine fluoride, and intelligence impairment of children in China: a school-based cross-sectional study. Ecotoxicol Environ Saf 165:270–277. https://doi.org/10.1016/j.ecoenv.2018.09.018
Zhao Q, Niu Q, Chen J, Xia T, Zhou G, Li P, Dong L, Xu C, Tian Z, Luo C, Liu L, Zhang S, Wang A (2019) Roles of mitochondrial fission inhibition in developmental fluoride neurotoxicity: mechanisms of action in vitro and associations with cognition in rats and children[J]. Arch Toxicol 93(3):709–726. https://doi.org/10.1007/s00204-019-02390-0
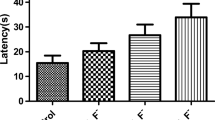
Zhou G, Tang S, Yang L, Niu Q, Chen J, Xia T, Wang S, Wang M, Zhao Q, Liu L, Li P, Dong L, Yang K, Zhang S, Wang A (2019) Effects of long-term fluoride exposure on cognitive ability and the underlying mechanisms: role of autophagy and its association with apoptosis[J]. Toxicol Appl Pharmacol 378:114608. https://doi.org/10.1016/j.taap.2019.114608
Lai C, Chen Q, Ding Y, Liu H, Tang Z (2020) Emodin protected against synaptic impairment and oxidative stress induced by fluoride in SH-SY5Y cells by modulating ERK1/2/Nrf2/HO-1 pathway[J]. Environ Toxicol 35(9):922–929. https://doi.org/10.1002/tox.22928
Ji F, Zhu Z, Zhang M, Zhang H, Zhu L, Cai X, Liu W, Song J, Li M, Cai Z (2020) 6-OH-BDE-47 exposure-induced Parkinson’s disease pathology in Sprague Dawley rat[J]. Sci Total Environ 711:135184. https://doi.org/10.1016/j.scitotenv.2019.135184
Pascual M, López-Hidalgo R, Montagud-Romero S, Ureña-Peralta JR, Rodríguez-Arias M, Guerri C (2021) Role of mTOR-regulated autophagy in spine pruning defects and memory impairments induced by binge-like ethanol treatment in adolescent mice[J]. Brain Pathol 31(1):174–188. https://doi.org/10.1111/bpa.12896
Zhang C, Feng X, He L, Zhang Y, Shao L (2020) The interrupted effect of autophagic flux and lysosomal function induced by graphene oxide in p62-dependent apoptosis of F98 cells[J]. J Nanobiotechnology 18(1):52. https://doi.org/10.1186/s12951-020-00605-6
Niu Q, Chen J, Xia T, Li P, Zhou G, Xu C, Zhao Q, Dong L, Zhang S, Wang A (2018) Excessive ER stress and the resulting autophagic flux dysfunction contribute to fluoride-induced neurotoxicity[J]. Environ Pollut 233:889–899. https://doi.org/10.1016/j.envpol.2017.09.015
Zhang C, Huo S, Fan Y, Gao Y, Yang Y, Sun D (2020) Autophagy may be involved in fluoride-induced learning impairment in rats[J]. Biol Trace Elem Res 193(2):502–507. https://doi.org/10.1007/s12011-019-01735-1
Lin J, Shi SS, Zhang JQ, Zhang YJ, Zhang L, Liu Y, Jin PP, Wei PF, Shi RH, Zhou W, Wen LP (2016) Giant cellular vacuoles induced by rare earth oxide nanoparticles are abnormally enlarged endo/lysosomes and promote mTOR-dependent TFEB nucleus translocation[J]. Small 12(41):5759–5768. https://doi.org/10.1002/smll.201601903
Lakpa KL, Khan N, Afghah Z, Chen X, Geiger JD (2021) Lysosomal stress response (LSR): physiological importance and pathological relevance[J]. J Neuroimmune Pharmacol 16(2):219–237. https://doi.org/10.1007/s11481-021-09990-7
Jeong SJ, Stitham J, Evans TD, Zhang X, Rodriguez-Velez A, Yeh YS, Tao J, Takabatake K, Epelman S, Lodhi IJ, Schilling JD, Debosch BJ, Diwan A, Razani B (2021) Trehalose causes low-grade lysosomal stress to activate TFEB and the autophagy-lysosome biogenesis response[J]. Autophagy 17(11):3740–3752. https://doi.org/10.1080/15548627.2021.1896906
Khan N, Lakpa KL, Halcrow PW, Afghah Z, Miller NM, Geiger JD, Chen X (2019) BK channels regulate extracellular Tat-mediated HIV-1 LTR transactivation[J]. Sci Rep 9(1):12285. https://doi.org/10.1038/s41598-019-48777-y
Mauthe M, Orhon I, Rocchi C, Zhou X, Luhr M, Hijlkema KJ, Coppes RP, Engedal N, Mari M, Reggiori F (2018) Chloroquine inhibits autophagic flux by decreasing autophagosome-lysosome fusion[J]. Autophagy 14(8):1435–1455. https://doi.org/10.1080/15548627.2018.1474314
Redmann M, Benavides GA, Berryhill TF, Wani WY, Ouyang X, Johnson MS, Ravi S, Barnes S, Darley-Usmar VM, Zhang J (2017) Inhibition of autophagy with bafilomycin and chloroquine decreases mitochondrial quality and bioenergetic function in primary neurons[J]. Redox Biol 11:73–81. https://doi.org/10.1016/j.redox.2016.11.004
Ye Y, Hui L, Lakpa KL, Xing Y, Wollenzien H, Chen X, Zhao JX, Geiger JD (2019) Effects of silica nanoparticles on endolysosome function in primary cultured neurons (1)[J]. Can J Physiol Pharmacol 97(4):297–305. https://doi.org/10.1139/cjpp-2018-0401
Koh JY, Kim HN, Hwang JJ, Kim YH, Park SE (2019) Lysosomal dysfunction in proteinopathic neurodegenerative disorders: possible therapeutic roles of cAMP and zinc[J]. Mol Brain 12(1):18. https://doi.org/10.1186/s13041-019-0439-2
Wang D, Cao L, Pan S, Wang G, Wang L, Cao N, Hao X (2021) Sirt3-mediated mitochondrial dysfunction is involved in fluoride-induced cognitive deficits[J]. Food Chem Toxicol 158:112665. https://doi.org/10.1016/j.fct.2021.112665
Nkpaa KW, Onyeso GI (2018) Rutin attenuates neurobehavioral deficits, oxidative stress, neuro-inflammation and apoptosis in fluoride treated rats[J]. Neurosci Lett 682:92–99. https://doi.org/10.1016/j.neulet.2018.06.023
Zhang C, Yang Y, Gao Y, Sun D (2022) NaF-induced neurotoxicity via activation of the IL-1β/JNK signaling pathway[J]. Toxicology 469:153132. https://doi.org/10.1016/j.tox.2022.153132
Snigdha S, Smith ED, Prieto GA, Cotman CW (2012) Caspase-3 activation as a bifurcation point between plasticity and cell death[J]. Neurosci Bull 28(1):14–24. https://doi.org/10.1007/s12264-012-1057-5
Tu W, Zhang Q, Liu Y, Han L, Wang Q, Chen P, Zhang S, Wang A, Zhou X (2018) Fluoride induces apoptosis via inhibiting SIRT1 activity to activate mitochondrial p53 pathway in human neuroblastoma SH-SY5Y cells[J]. Toxicol Appl Pharmacol 347:60–69. https://doi.org/10.1016/j.taap.2018.03.030
Zatyka M, Sarkar S, Barrett T (2020) Autophagy in Rare (NonLysosomal) Neurodegenerative Diseases[J]. J Mol Biol 432(8):2735–2753. https://doi.org/10.1016/j.jmb.2020.02.012
Filali-Mouncef Y, Hunter C, Roccio F et al (2022) The ménage à trois of autophagy, lipid droplets and liver disease[J]. Autophagy 18(1):50–72. https://doi.org/10.1080/15548627.2021.1895658
Bjørkøy G, Lamark T, Pankiv S, Øvervatn A, Brech A, Johansen T (2009) Monitoring autophagic degradation of p62/SQSTM1[J]. Methods Enzymol 452:181–197. https://doi.org/10.1016/s0076-6879(08)03612-4
Seibenhener ML, Babu JR, Geetha T, Wong HC, Krishna NR, Wooten MW (2004) Sequestosome 1/p62 is a polyubiquitin chain binding protein involved in ubiquitin proteasome degradation[J]. Mol Cell Biol 24(18):8055–8068. https://doi.org/10.1128/mcb.24.18.8055-8068.2004
González-Rodríguez A, Mayoral R, Agra N, Valdecantos MP, Pardo V, Miquilena-Colina ME, Vargas-Castrillón J, Lo Iacono O, Corazzari M, Fimia GM, Piacentini M, Muntané J, Boscá L, García-Monzón C, Martín-Sanz P, Valverde ÁM (2014) Impaired autophagic flux is associated with increased endoplasmic reticulum stress during the development of NAFLD[J]. Cell Death Dis 5(4):e1179. https://doi.org/10.1038/cddis.2014.162
Stead ER, Castillo-Quan JI, Miguel VEM, Lujan C, Ketteler R, Kinghorn KJ, Bjedov I (2019) Agephagy - adapting autophagy for health during aging[J]. Front Cell Dev Biol 7:308. https://doi.org/10.3389/fcell.2019.00308
Emanuel R, Sergin I, Bhattacharya S, Turner J, Epelman S, Settembre C, Diwan A, Ballabio A, Razani B (2014) Induction of lysosomal biogenesis in atherosclerotic macrophages can rescue lipid-induced lysosomal dysfunction and downstream sequelae[J]. Arterioscler Thromb Vasc Biol 34(9):1942–1952. https://doi.org/10.1161/atvbaha.114.303342
Sergin I, Evans TD, Zhang X, Bhattacharya S, Stokes CJ, Song E, Ali S, Dehestani B, Holloway KB, Micevych PS, Javaheri A, Crowley JR, Ballabio A, Schilling JD, Epelman S, Weihl CC, Diwan A, Fan D, Zayed MA, Razani B (2017) Exploiting macrophage autophagy-lysosomal biogenesis as a therapy for atherosclerosis[J]. Nat Commun 8:15750. https://doi.org/10.1038/ncomms15750
Yoo YJ, Kim H, Park SR et al (2017) An overview of rapamycin: from discovery to future perspectives[J]. J Ind Microbiol Biotechnol 44(4–5):537–553. https://doi.org/10.1007/s10295-016-1834-7
Zhang Y, Han X, Tang Y, Zhang J, Hu Z, Xu W, Yao P, Niu Q (2021) Weakened interaction of ATG14 and the SNARE complex blocks autophagosome-lysosome fusion contributes to fluoride-induced developmental neurotoxicity[J]. Ecotoxicol Environ Saf 230:113108. https://doi.org/10.1016/j.ecoenv.2021.113108
Yamamoto T, Takabatake Y, Takahashi A, Kimura T, Namba T, Matsuda J, Minami S, Kaimori JY, Matsui I, Matsusaka T, Niimura F, Yanagita M, Isaka Y (2017) High-fat diet-induced lysosomal dysfunction and impaired autophagic flux contribute to lipotoxicity in the kidney[J]. J Am Soc Nephrol 28(5):1534–1551. https://doi.org/10.1681/asn.2016070731
Park HS, Song JW, Park JH, Lim BK, Moon OS, Son HY, Lee JH, Gao B, Won YS, Kwon HJ (2021) TXNIP/VDUP1 attenuates steatohepatitis via autophagy and fatty acid oxidation[J]. Autophagy 17(9):2549–2564. https://doi.org/10.1080/15548627.2020.1834711
Yang H, Wen Y, Zhang M, Liu Q, Zhang H, Zhang J, Lu L, Ye T, Bai X, Xiao G, Wang M (2020) MTORC1 coordinates the autophagy and apoptosis signaling in articular chondrocytes in osteoarthritic temporomandibular joint. Autophagy 16(2):271–288. https://doi.org/10.1080/15548627.2019.1606647
Funding
This work was supported by grants from the National Natural Science Foundation of China (Grant No. 81860559 and Grant No. 82060580), the Program of Science and Technology Innovation in Bingtuan (Grant No. 2021CB046), and the High-Level Talent Research Project of Shihezi University (Grant No. RCZK2018C02).
Author information
Authors and Affiliations
Contributions
Wanjing Xu and Zeyu Hu: conceptualization; methodology; data curation; writing—original draft preparation. Yanling Tang: visualization, investigation. Jingjing Zhang: supervision. Shangzhi X: software, validation. Qiang Niu: writing—reviewing and editing.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xu, W., Hu, Z., Tang, Y. et al. Excessive Lysosomal Stress Response and Consequently Impaired Autophagy Contribute to Fluoride-Induced Developmental Neurotoxicity. Biol Trace Elem Res 201, 4472–4483 (2023). https://doi.org/10.1007/s12011-022-03511-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03511-0