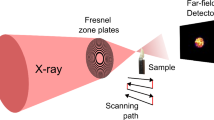
Abstract
The human neocortex has a cytoarchitecture composed of six layers with an intrinsic organization that relates to afferent and efferent pathways for a high functional specialization. Various histological, neurochemical, and connectional techniques have been used to study these cortical layers. Here, we explore the additional possibilities of swift ion beam and synchrotron radiation techniques to distinguish cellular layers based on the elemental distributions and areal density pattern in the human neocortex. Temporal cortex samples were obtained from two neurologically normal adult men (postmortem interval: 6–12 h). A cortical area of 500 × 500 μm2 was scanned by a 3 MeV proton beam for elemental composition and areal density measurements using particle induced x-ray emission (PIXE) and scanning transmission ion microscopy (STIM), respectively. Zinc showed higher values in cortical layers II and V, which needs a critical discussion. Furthermore, the areal density decreased in regions with a higher density of pyramidal neurons in layers III and V. Scanning transmission X-ray microscopy (STXM) revealed the cellular density with higher lateral resolution than STIM, but not enough to distinguish each cortical lamination border. Our data describe the practical results of these approaches employing both X-ray and ion-beam based techniques for the human cerebral cortex and its heterogeneous layers. These results add to the potential approaches and knowledge of the human neocortical gray matter in normal tissue to develop improvements and address further studies on pathological conditions.





Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the conditions and rules of work of the laboratories where the samples were analyzed but are available from the corresponding author on reasonable request.
References
Nieuwenhuys R, Voogd J, van Huijzen CHR (1988) The human central nervous system. Springer-Verlag, Berlin
Kolb B, Whishaw IQ (2021) Fundamentals of human neuropsychology. Worth Publishers, New York
Rasia-Filho AA, Guerra KTK, Vásquez CE, Dall’Oglio A, Reberger R, Jung CR, Calcagnotto ME (2021) The subcortical-allocortical-neocortical continuum for the emergence and morphological heterogeneity of pyramidal neurons in the human brain. Front Synaptic Neurosci 13:607–616
Pandya DN, Seltzer B, Petrides M, Cipolloni PB (2015) Cerebral cortex – architecture, connections, and the dual origin concept. Oxford University Press, New York
Shepherd GM, Rowe TB (2017) Neocortical lamination: insights from neuron types and evolutionary precursors. Front Neuroanat 11(100):1–7
Scholtens L, Schmidt HR, de Reus MA, van den Heuvel MP (2014) Linking macroscale graph analytical organization to microscale neuroarchitectonics in the macaque connectome. J Neurosci 34(36):12192–12205
Guy J, Staiger JF (2017) The functioning of a cortex without layers. Front Neuroanat 11(54):1–13
Buxhoeveden DP, Casanova MF (2002) The minicolumn hypothesis in neuroscience. Brain 125:935–951
Palomero-Gallagher N, Zilles K (2017) Cortical layers: cyto-, myelo-, receptor- and synaptic architecture in human cortical areas. Neuroimage S1053–8119:30682–30691
DeFelipe J (2011) The evolution of the brain, the human nature of cortical circuits, and intellectual creativity. Front Neuroanat 5(29):1–17
Gahr M (1997) How should brain nuclei be delineated? Consequences for developmental mechanisms and for correlations of area size, neuron numbers and functions of brain nuclei. Trends Neurosci 20:58–62
Lanciego JL, Wouterlood FG (2011) A half century of experimental neuroanatomical tracing. J Chem Neuroanat 42(3):157–183
Hodge RD, Miller JA, Novotny M, Kalmbach BE, Ting JT, Bakken TE, Aevermann BD, Barkan ER, Berkowitz-Cerasano ML, Cobbs C, Diez-Fuertes F, Ding SL, McCorrison J, Schork NJ, Shehata SI, Smith KA, Sunkin SM, Tran DN, Venepally P, Yanny AM, Steemers FJ, Phillips JW, Bernard A, Koch C, Lasken RS, Scheuermann RH, Lein ES (2020) Transcriptomic evidence that von Economo neurons are regionally specialized extratelencephalic-projecting excitatory neurons. Nat Commun 11(1):1172
Johansson SAE, Campbell JL, Malmqvist KG (1995) Particle-induced X-ray emission spectrometry (PIXE). John Wiley, New York
Stori EM, Souza CT, Dias JF (2016) Fabrication and analysis of polymer microstructures through ion microprobe techniques. J App Poly Sci 43253:1–10
Jobim PFC, dos Santos CEI, Maurmann N, Reolon G, Debastiani R, Pedroso TR, Carvalho LM, Dias JF (2014) Analysis of memory consolidation and evocation in rats by proton induced X-ray emission. Nucl Instrum Methods Phys Res B 332:224–228
Jobim PFC, dos Santos CEI, Jeromel L, Pellicon P, Amaral L, Dias JF (2018) Changes in the element concentration of the dorsal hippocampus CA1 region during memory consolidation and reconsolidation. J Chem Neuroanat 90:49–56
Zhang B, Ren M, Sheu FS, Watt F, Routtenberg A (2012) Quantitative analysis of zinc in rat hippocampal mossy fibers by nuclear microscopy. Neuros Res 74:17–24
Boruchowska M, Lankosz M, Adamek D, Korman A (2001) PIXE analysis of human brain tissue. X Ray Spec 30:174–179
Tilko G, Mesjasz-Przybyłowicz J, Przybyłowicz WJ (2007) X-ray microanalysis of biological material in the frozen-hydrated state by PIXE. Micros Res Tech 70:55–68
Stori EM, Souza CT, Amaral L, Fink D, Papaleo RM, Dias JF (2013) Use of STIM for morphological studies of microstructured polymer foils. Nuc Instrum Meth Phys Res 306:99–103
Pascolo L, Gianoncelli A, Kaulich B, Rizzardi C, Schneider M, Bottin C, Polentarutti M, Kiskinova M, Longoni A, Melato M (2011) Synchrotron soft X-ray imaging and fluorescence microscopy reveal novel features of asbestos body morphology and composition in human lung tissues. Part Fibre Toxicol 8(7):1–11
Pascolo L, Gianoncelli A, Schneider G, Salome M, Schneider M, Calligaro C, Kiskinova M, Melato M, Rizzardi C (2013) The interaction of asbestos and iron in lung tissue revealed by synchrotron-based scanning X-ray microscopy. Sci Rep 3(1123):1–11
Gianoncelli A, Kourousias G, Merolle L, Altissimo M, Bianco A (2016) Combining multiple imaging techniques at the TwinMic X-ray microscopy beamline. J Synchrotron Radiat 23(60):1526–1537
Krebs N, Langkammer C, Goessler W, Ropelec S, Fazekas F, Yene K, Scheurer E (2014) Assessment of trace elements in human brain using inductively coupled plasma mass spectrometry. J Trace Elem Med Biol 28:1–7
Lins BR, Pushie JM, Jones M, Howard DL, Howland JG, Hackett MJ (2016) Mapping alterations to the endogenous elemental distribution within the lateral ventricles and choroid plexus in brain disorders using X-ray fluorescence imaging. PLoS ONE 11(6):e0158152. https://doi.org/10.1371/journal.pone.0158152
Mai JK, Paxinos G, Voss T (2008) Atlas of the human brain. Academic Press, New York
Al-Ebraheem A, Dao E, Desouza E, Li C, Wainman BC, McNeill FE, Farquharson MJ (2015) Effect of sample preparation techniques on the concentrations and distributions of elements in biological tissues using μSRXRF: a comparative study. Physiol Meas 36:N51
Ziegler J (2008) SRIM is a collection of software packages which calculate many features of the transport of ions in matter. https://www.srim.org. Accessed 11 October 2011
Fernandes F, Niedraszewicz LAB, Amaral L, Dias JF (2018) Evaluation of detector efficiency through GUPIXWIN H value. Nucl Instrum Methods Phys Res B 417:56–59
Campbell JL, Boyd NI, Grassi N, Bonnick P, Maxwell JA (2010) The Guelph PIXE software package IV. Nucl Instrum Methods Phys Res B 268:3356–3363
Ryan CG (2000) Quantitative trace element imaging using PIXE and the nuclear microprobe. Imag Syst Tech 11:219–230
Grime J (2017) Equipment and software for high energy ion microbeam applications OMDAQ. http://www.microbeams.co.uk/download.html. Accessed 20 May 2018
Lobinski R, Moulin C, Ortega R (2006) Imaging and speciation of trace elements in biological environment. Biochimie 88:1591–1604
Gigineishvili TV, Gegenava LG, Machavariani NA, Magradze NM (2007) Variations in the content of some trace elements and macroelements in the hippocampus of rats during learning and memory retrieval after destruction of the entorhinal cortex. Bull Exp Bio Med 143(6):667–669
Serpa RFB, de Jesus EFO, Anjos MJ, de Oliveira LF, Marins LA, do Carmo MGT, Corrêa Junior JD, Rocha MS, Lopes RT, Martinez AMB (2008) Topographic trace-elemental analysis in the brain of Wistar rats by X-ray microfluorescence with synchrotron radiation. Anal Sci 24:839–842
Boruchowska M, Lankosz M, Adamek D, Ostachowicz B, Ostachowicz J, Tomik B (2002) X-ray fluorescence analysis of human brain tissue and body fluids. Polish J Med Phys Eng 8(3):173–181
Rollenhagen A, Walkenfort B, Yakoubi R, Klauke SA, Schmuhl-Giesen SF, Heinen-Weiler J, Voortmann S, Marshallsay B, Palaz T, Holz U, Hasenberg M, Lübke JHR (2020) Synaptic organization of the human temporal lobe neocortex as revealed by high-resolution transmission, focused ion beam scanning, and electron microscopic tomography. Int J Mol Sci 21(15):5558, 1–27
Montero-Crespo M, Dominguez-Alvaro M, Rondon-Carrillo P, Alonso-Nanclares L, DeFelipe J, Blazquez-Llorca L (2020) Three-dimensional synaptic organization of the human hippocampal CA1 field. Elife 9:e57013
Assaf Y (2017) Imaging laminar structures in the gray matter with diffusion MRI. Neuroimage 17:31120–31125
Mesjasz-Przybyłowicz J, Przybyłowicz WJ (2002) Micro-PIXE in plant sciences: present status and perspectives. Nucl Inst Met Phys Res B 189:470–481
Vogel-Mikus K, Pongrac O, Primoz P (2015) Micro-PIXE elemental mapping for ionome studies of crop plants. Int J PIXE 249(3):217–233
Chwiej J, Szczerbowska-Boruchowska M, Lankosz M, Wojcik S, Falkenberg G, Stegowski Z, Setkowicz Z (2005) Preparation of tissue samples for X-ray fluorescence microscopy. Spectrochim Acta Part B: At Spectrosc 60(12):1531–1537
Pushie MJ, Pickering IJ, Korbas M, Hackett MJ, George GN (2014) Elemental and chemically specific X-ray fluorescence imaging of biological systems. Chem Rev 114:8499–8541
Pushie MJ, Hollings A, Reinhardt J, Webb SM, Lam V, Takechi R, Mamo JC, Paterson PG, Kelly ME, George GN, Pickering IJ, Hackett MJ (2020) Sample preparation with sucrose cryoprotection dramatically alters Zn distribution in the rodent hippocampus, as revealed by elemental mapping. J Anal At Spectrom 35(11):2498–2508
Gellein K, Flaten TP, Erikson KM, Aschner M, Syversen T (2008) Leaching of trace elements from biological tissue by formalin fixation. Biol Trace Elem Res 121(3):221–225
Alaverdashvili M, Hackett MJ, Pickering IJ, Paterson PG (2014) Laminar-specific distribution of zinc: evidence for presence of layer IV in forelimb motor cortex in the rat. Neuroimage 103:502–510
Balaram P, Young NA, Kaas JH (2014) Histological features of layers and sublayers in cortical visual areas V1 and V2 of chimpanzees, macaque monkeys, and humans. Eye Brain 6(1):5–18
Cunha MML, Trepout S, Messaoudi C, Wu T, Ortega R, Guerquin-Kern J, Marco S (2016) Overview of chemical imaging methods to address biological questions. Micron 84:23–36
Hackett MJ, McQuillan AJ, El-Assaad F, Aitken JB, Levina A, Cohen DD, Siegele R, Carter EA, Georges EG, Grau GE, Hunt NH, Lay PA (2011) Chemical alterations to murine brain tissue induced by formalin fixation: implications for biospectroscopic imaging and mapping studies of disease pathogenesis. Analyst 136:2941–2952
Bush VJ, Moyer TP, Batts KP, Parisi JE (1995) Essential and toxic element concentrations in fresh and formalin-fixed human autopsy tissues. Clin Chem 4(2):284–294
Tran H, Jan N, Hu D, Voorhees A, Schuman JS, Smith MA, Wollstein G, Sigal IA (2017) Formalin fixation and cryosectioning cause only minimal changes in shape or size of ocular tissues. Sci Rep 7(12065):1–11
Khalil R, Levitt JB (2017) Use of synaptic zinc histochemistry to reveal different regions and laminae in the developing and adult brain. J Vis Exp 128:e56547
Vergnano AM, Rebola N, Savtchenko LP, Pinheiro OS, Casado M, Kieffer BL, Rusakov DA, Mulle C, Paoletti P (2014) Zinc dynamics and action at excitatory synapses. Neuron 82(5):1101–1114
Dall’Oglio A, Xavier LL, Hilbig A, Ferme D, Moreira JE, Achaval M, Rasia-Filho AA (2013) Cellular components of the human medial amygdaloid nucleus. J Comp Neurol 521:589–611
Vásquez CE, Reberger R, Dall’Oglio A, Calcagnotto ME, Rasia-Filho AA (2018) Neuronal types of the human cortical amygdaloid nucleus. J Comp Neurol 526:2776–2801
Lemelle L, Simionovici A, Colin P, Knott G, Bohic S, Cloetens P, Schneider BL (2020) Nano-imaging trace elements at organelle levels in substantia nigra overexpressing α-synuclein to model Parkinson’s disease. Commun Biol 3:364. https://doi.org/10.1038/s42003-020-1084-0
Massimi L, Bukreeva I, Santamaria G, Fratini M, Corbelli A, Brun F, Fumagalli S, Maugeri L, Pacureanu A, Cloetens P, Pieroni N, Fiordaliso F, Forloni G, Uccelli A, Rosbo NK, Balducci C, Cedola A (2019) Exploring Alzheimer’s disease mouse brain through X-ray phase contrast tomography: from the cell to the organ. Neuroimage 184:490–495
Hare DJ, New EJ, de Jongee MD, McColl G (2015) Imaging metals in biology: balancing sensitivity, selectivity and spatial resolution. Chem Soc Rev. https://doi.org/10.1039/c5cs00055f
Hare DJ, New EJ (2016) On the outside looking in: redefining the role of analytical chemistry in the biosciences. Chem Commun. https://doi.org/10.1039/c6cc00128a
New EJ, Wimmer VC, Hare DJ (2017) Promises and pitfalls of metal imaging in biology. Cell Chem Biol. https://doi.org/10.1016/j.chembiol.2017.10.006
Miller LM, Dumas P (2006) Chemical imaging of biological tissue with synchrotron infrared light. Bioch Biophys Acta 1758:846–857
Magnain C, Augustinack JC, Tirrell L, Fogarty M, Frosch MP, Boas D, Fischl B, Rockland KS (2019) Colocalization of neurons in optical coherence microscopy and Nissl-stained histology in Brodmann’s area 32 and area 21. Brain Struct Funct 224(1):351–362
Acknowledgements
The authors thank Dr. Gabriela Sena (UERJ, Brazil), Dr. Anja Kavčič (University of Ljubljana, Slovenia), and Mrs. Carla Calligaro (University of Udine, Italy) for the support during sample preparation for STXM and microPIXE.
Funding
To perform the research reported in this manuscript, Paulo Jobim received a Brazilian CNPq post-doctoral grant (# 150037/2017–1) and Carla dos Santos a CAPES fellowship grant (# POS-DOC 88881.119418/2016–01). Work at JSI was supported by the ARRS Grants J7-9398, N1-0090, P1-0112, I0-0005 and EU H2020 Project No. 824096 “RADIATE”.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, measurements, data collection and analysis, and manuscript preparation were performed by Paulo Fernandes Costa Jobim and Carla Eliete Iochims dos Santos; Alberto Antônio Rasia-Filho performed the sample collection and collaborated in the data analysis and manuscript preparation; Johnny Ferraz Dias contributed to the measurements, data analysis, and manuscript; Mitja Kelemen, Primož Pelicon, Katarina Vogel Mikuš, Lorella Pascolo, Alessandra Gianoncelli, and Diana Eva Bedolla contributed to the sample preparation, measurements, and data analysis. The first draft of the manuscript was written by Paulo Fernandes Costa Jobim and all the authors commented on the previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics Approval
This study followed the ethical procedures in accordance with international regulatory standards (based on the 1964 Helsinki Declaration) and were approved by the Ethics Committees of the Federal University of Health Sciences of Porto Alegre (Brazil; “Plataforma Brasil” process # 62381916.9.0000.5345/2018), and the Federal University of Rio Grande do Sul (Brazil; “Plataforma Brasil” process # 62381916.9.3001.5347/2017).
Consent to Participate
The next of kin provided written informed consent for brain donation and for use in this kind of study. Informed consent and general clinical data on known comorbidities were obtained from the next of kin at the morgue.
Consent for Publication
There is no potentially identifiable data for any individual included in this article.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jobim, P.F.C., Iochims dos Santos, C.E., Dias, J.F. et al. Human Neocortex Layer Features Evaluated by PIXE, STIM, and STXM Techniques. Biol Trace Elem Res 201, 592–602 (2023). https://doi.org/10.1007/s12011-022-03182-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-022-03182-x