Abstract
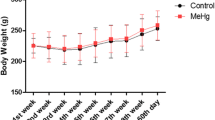
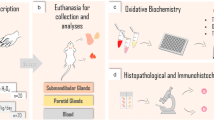
Methylmercury (MeHg) is one of the main global pollutants. The vulnerability of fetus and newborn to MeHg-induced changes is extensively reported, making relevant investigation possible for alternative sample matrix for human biological monitoring for at this stage of life. This study aimed to characterize tissue change effects of environmental–experimental MeHg on salivary glands of offspring rats after pre- and postnatal exposure. For this, pregnant Wistar rats were orally exposed to MeHg (40 μg/kg BW/day) or only vehicle (control group), from the gestational period to the end of the lactation period. Salivary glands (SG) were collected from the offspring to analyze possible Hg levels and main findings by histopathological evaluations and CK19 and α-SMA immunostaining. The results indicated that Hg levels in SG of intoxicated offspring were associated with histologic abnormalities, such as acinar atrophy and an increase in the intercellular matrix among the acini, as well as damages in the architecture of epithelium and myoepithelial cells, evidenced by a decrease in immunostaining area. Thus, this is the first study to show in the literature the toxicopathologic findings on SG of offspring after pre- and postnatal exposure to MeHg. Moreover, it presents the SG as an attractive target to futures studies, mainly in children exposed to environmentally relevant doses.




Similar content being viewed by others
Data Availability
The quantitative and qualitative data used to support the findings of this study are included within the article.
References
Clarkson TW, Magos L (2006) The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36(8):609–662. https://doi.org/10.1080/10408440600845619
Posin SL, Sharma S (2020) Mercury toxicity. In: StatPearls. StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC, Treasure Island (FL)
UN Environment (2019) Global mercury assessment 2018. ISBN: 978-92-807-3744-8
Bjørklund G, Dadar M, Mutter J, Aaseth J (2017) The toxicology of mercury: current research and emerging trends. Environ Res 159:545–554. https://doi.org/10.1016/j.envres.2017.08.051
Gebeyehu HR, Bayissa LD (2020) Levels of heavy metals in soil and vegetables and associated health risks in Mojo area, Ethiopia. PLoS One 15(1):e0227883. https://doi.org/10.1371/journal.pone.0227883
Esdaile LJ, Chalker JM (2018) The mercury problem in artisanal and small-scale gold mining. 24(27):6905-6916. https://doi.org/10.1002/chem.201704840
Berzas Nevado JJ, Rodríguez Martín-Doimeadios RC, Guzmán Bernardo FJ, Jiménez Moreno M, Herculano AM, do Nascimento JL, Crespo-López ME (2010) Mercury in the Tapajós River basin, Brazilian Amazon: a review. Environ Int 36(6):593–608. https://doi.org/10.1016/j.envint.2010.03.011
Bank MS (2020) The mercury science-policy interface: history, evolution and progress of the Minamata Convention. Sci Total Environ 722:137832. https://doi.org/10.1016/j.scitotenv.2020.137832
Hong C, Yu X, Liu J, Cheng Y, Rothenberg SE (2016) Low-level methylmercury exposure through rice ingestion in a cohort of pregnant mothers in rural China. Environ Res 150:519–527. https://doi.org/10.1016/j.envres.2016.06.038
Rodríguez Martín-Doimeadios RC, Berzas Nevado JJ, Guzmán Bernardo FJ, Jiménez Moreno M, Arrifano GPF, Herculano AM, do Nascimento JLM, Crespo-López ME (2014) Comparative study of mercury speciation in commercial fishes of the Brazilian Amazon. Environ Sci Pollut Res 21(12):7466–7479. https://doi.org/10.1007/s11356-014-2680-7
Buck DG, Evers DC, Adams E, DiGangi J, Beeler B, Samánek J, Petrlik J, Turnquist MA, Speranskaya O, Regan K, Johnson S (2019) A global-scale assessment of fish mercury concentrations and the identification of biological hotspots. Sci Total Environ 687:956–966. https://doi.org/10.1016/j.scitotenv.2019.06.159
Rice KM, Walker EM Jr, Wu M, Gillette C, Blough ER (2014) Environmental mercury and its toxic effects. J Prev Med Public Health 47(2):74–83. https://doi.org/10.3961/jpmph.2014.47.2.74
Ceccatelli S, Bose R, Edoff K, Onishchenko N, Spulber S (2013) Long-lasting neurotoxic effects of exposure to methylmercury during development. J Intern Med 273(5):490–497. https://doi.org/10.1111/joim.12045
Ursinyova M, Masanova V, Uhnakova I, Murinova LP, Patayova H, Rausova K, Trnovec T, Stencl J, Gajdos M (2019) Prenatal and early postnatal exposure to total mercury and methylmercury from low maternal fish consumption. Biol Trace Elem Res 191(1):16–26. https://doi.org/10.1007/s12011-018-1585-6
Rocha JB, Aschner M, Dórea JG, Ceccatelli S, Farina M, Silveira LC (2012) Mercury toxicity. J Biomed Biotechnol 2012:831890–831892. https://doi.org/10.1155/2012/831890
Björnberg KA, Vahter M, Berglund B, Niklasson B, Blennow M, Sandborgh-Englund G (2005) Transport of methylmercury and inorganic mercury to the fetus and breast-fed infant. Environ Health Perspect 113(10):1381–1385. https://doi.org/10.1289/ehp.7856
Bittencourt LO, Puty B, Charone S, Aragão WAB, Farias-Junior PM, Silva MCF, Crespo-Lopez ME, Leite AL, Buzalaf MAR, Lima RR (2017) Oxidative biochemistry disbalance and changes on proteomic profile in salivary glands of rats induced by chronic exposure to methylmercury. Oxidative Med Cell Longev 2017:5653291–5653215. https://doi.org/10.1155/2017/5653291
Farias-Junior PMA, Teixeira FB, Fagundes NCF, Miranda GHN, Oliveira Bittencourt L, de Oliveira Paraense RS, Silva MCF, Sagica F, de Oliveira EH, Crespo-López ME, Lima RR (2017) Chronic intoxication by methylmercury leads to oxidative damage and cell death in salivary glands of rats. Metallomics 9(12):1778–1785. https://doi.org/10.1039/c7mt00168a
Lima LAO, Bittencourt LO, Puty B, Fernandes RM, Nascimento PC, Silva MCF, Alves-Junior SM, Pinheiro JJV, Lima RR (2018) Methylmercury intoxication promotes metallothionein response and cell damage in salivary glands of rats. Biol Trace Elem Res 185(1):135–142. https://doi.org/10.1007/s12011-017-1230-9
Maruyama CL, Monroe MM, Hunt JP, Buchmann L, Baker OJ (2019) Comparing human and mouse salivary glands: a practice guide for salivary researchers. Oral Dis 25(2):403–415. https://doi.org/10.1111/odi.12840
Kingsnorth AN, Skandalakis PN, Colborn GL, Weidman TA, Skandalakis LJ, Skandalakis JE (2000) Embryology, anatomy, and surgical applications of the preperitoneal space. Surg Clin North Am 80(1):1–24. https://doi.org/10.1016/s0039-6109(05)70394-7
Michalke B, Rossbach B, Göen T, Schäferhenrich A, Scherer G (2015) Saliva as a matrix for human biomonitoring in occupational and environmental medicine. Int Arch Occup Environ Health 88(1):1–44. https://doi.org/10.1007/s00420-014-0938-5
Caporossi L, Santoro A, Papaleo B (2010) Saliva as an analytical matrix: state of the art and application for biomonitoring. Biomarkers 15(6):475–487. https://doi.org/10.3109/1354750x.2010.481364
Council NR (2011) Guide for the care and use of laboratory animals, Eighth edn. The National Academies Press, Washington, DC. https://doi.org/10.17226/12910
Kilkenny C, Browne WJ, Cuthill IC, Emerson M, Altman DG (2010) Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol 8(6):e1000412. https://doi.org/10.1371/journal.pbio.1000412
Kong HK, Wong MH, Chan HM, Lo SC (2013) Chronic exposure of adult rats to low doses of methylmercury induced a state of metabolic deficit in the somatosensory cortex. J Proteome Res 12(11):5233–5245. https://doi.org/10.1021/pr400356v
Liang J, Inskip M, Newhook D, Messier C (2009) Neurobehavioral effect of chronic and bolus doses of methylmercury following prenatal exposure in C57BL/6 weanling mice. Neurotoxicol Teratol 31(6):372–381. https://doi.org/10.1016/j.ntt.2009.08.007
Kirkpatrick M, Benoit J, Everett W, Gibson J, Rist M, Fredette N (2015) The effects of methylmercury exposure on behavior and biomarkers of oxidative stress in adult mice. Neurotoxicology 50:170–178. https://doi.org/10.1016/j.neuro.2015.07.001
Taga R, Sesso A (1979) Ultrastructural studies on developing parotid gland of the rat at early postnatal periods. Arch Histol Jpn 42(4):427–444. https://doi.org/10.1679/aohc1950.42.427
Santana L, Bittencourt LO, Nascimento PC, Fernandes RM, Teixeira FB, Fernandes LMP, Freitas Silva MC, Nogueira LS, Amado LL, Crespo-Lopez ME, Maia C, Lima RR (2019) Low doses of methylmercury exposure during adulthood in rats display oxidative stress, neurodegeneration in the motor cortex and lead to impairment of motor skills. J Trace Elem Med Biol 51:19–27. https://doi.org/10.1016/j.jtemb.2018.09.004
Bohl L, Merlo C, Carda C, Gomez de Ferraris ME, Carranza M (2008) Morphometric analysis of the parotid gland affected by alcoholic sialosis. J Oral Pathol Med 37(8):499–503. https://doi.org/10.1111/j.1600-0714.2008.00648.x
Chu PG, Weiss LM (2002) Keratin expression in human tissues and neoplasms. Histopathology 40(5):403–439. https://doi.org/10.1046/j.1365-2559.2002.01387.x
Ogawa Y (2003) Immunocytochemistry of myoepithelial cells in the salivary glands. Prog Histochem Cytochem 38(4):343–426. https://doi.org/10.1016/S0079-6336(03)80001-3
Ronchetti R, Zuurbier M, Jesenak M, Koppe JG, Ahmed UF, Ceccatelli S, Villa MP (2006) Children’s health and mercury exposure. 95(s453):36-44. https://doi.org/10.1080/08035250600886157
Branch WUDC (2008) Guidance for identifying populations at risk from mercury exposure.
de Paula Fonseca Arrifano G, Del Carmen Rodriguez Martin-Doimeadios R, Jiménez-Moreno M, Augusto-Oliveira M, Rogério Souza-Monteiro J, Paraense R, Rodrigues Machado C, Farina M, Macchi B, do Nascimento JLM, Crespo-Lopez ME (2018) Assessing mercury intoxication in isolated/remote populations: increased S100B mRNA in blood in exposed riverine inhabitants of the Amazon. Neurotoxicology 68:151–158. https://doi.org/10.1016/j.neuro.2018.07.018
Santos Serrão de Castro N, de Oliveira Lima M (2018) Hair as a biomarker of long term mercury exposure in Brazilian Amazon: a systematic review. Int J Environ Res Public Health 15(3). https://doi.org/10.3390/ijerph15030500
Arrifano GPF, Martín-Doimeadios RCR, Jiménez-Moreno M, Fernández-Trujillo S, Augusto-Oliveira M, Souza-Monteiro JR, Macchi BM, Alvarez-Leite JI, do Nascimento JLM, Amador MT, Santos S, Ribeiro-Dos-Santos Â, Silva-Pereira LC, Oriá RB, Crespo-Lopez ME (2018) Genetic susceptibility to neurodegeneration in Amazon: apolipoprotein E genotyping in vulnerable populations exposed to mercury. Front Genet 9:285. https://doi.org/10.3389/fgene.2018.00285
Arrifano GPF, Martín-Doimeadios RCR, Jiménez-Moreno M, Ramírez-Mateos V, da Silva NFS, Souza-Monteiro JR, Augusto-Oliveira M, Paraense RSO, Macchi BM, do Nascimento JLM, Crespo-Lopez ME (2018) Large-scale projects in the Amazon and human exposure to mercury: the case-study of the Tucuruí Dam. Ecotoxicol Environ Saf 147:299–305. https://doi.org/10.1016/j.ecoenv.2017.08.048
Council NR (2000) Toxicological effects of methylmercury. The National Academies Press, Washington, DC. https://doi.org/10.17226/9899
Al-Saleh I, Abduljabbar M, Al-Rouqi R, Elkhatib R, Alshabbaheen A, Shinwari N (2013) Mercury (Hg) exposure in breast-fed infants and their mothers and the evidence of oxidative stress. Biol Trace Elem Res 153(1-3):145–154. https://doi.org/10.1007/s12011-013-9687-7
Day JJ, Reed MN, Newland MC (2005) Neuromotor deficits and mercury concentrations in rats exposed to methyl mercury and fish oil. Neurotoxicol Teratol 27(4):629–641. https://doi.org/10.1016/j.ntt.2005.03.011
Gervais EM, Sequeira SJ, Wang W, Abraham S, Kim JH, Leonard D, DeSantis KA, Larsen M (2016) Par-1b is required for morphogenesis and differentiation of myoepithelial cells during salivary gland development. Organogenesis 12(4):194–216. https://doi.org/10.1080/15476278.2016.1252887
Porcheri C, Mitsiadis TA (2019) Physiology, pathology and regeneration of salivary glands. Cells 8(9):976. https://doi.org/10.3390/cells8090976
Wells KL, Gaete M, Matalova E, Deutsch D, Rice D, Tucker AS (2013) Dynamic relationship of the epithelium and mesenchyme during salivary gland initiation: the role of Fgf10. Biol Open 2(10):981–989. https://doi.org/10.1242/bio.20135306
Sisto M, Lisi S, Ribatti D (2018) The role of the epithelial-to-mesenchymal transition (EMT) in diseases of the salivary glands. Histochem Cell Biol 150(2):133–147. https://doi.org/10.1007/s00418-018-1680-y
Millsop JW, Wang EA, Fazel N (2017) Etiology, evaluation, and management of xerostomia. Clin Dermatol 35(5):468–476. https://doi.org/10.1016/j.clindermatol.2017.06.010
Epstein JB, Villines DC, Sroussi HY (2015) Oral symptoms and oral function in people with Sjögren’s syndrome. Clin Exp Rheumatol 33(1):132–133
Mauri-Obradors E, Estrugo-Devesa A, Jané-Salas E, Viñas M, López-López J (2017) Oral manifestations of diabetes mellitus. A systematic review. Med Oral Patol Oral Cir Bucal 22(5):e586–e594. https://doi.org/10.4317/medoral.21655
Talha B, Swarnkar SA (2020) Xerostomia. In: StatPearls. StatPearls Publishing Copyright © 2020, StatPearls Publishing LLC, Treasure Island (FL)
Acknowledgments
The authors would like to thank the Federal University of Pará and Federal University of Rio Grande do Sul for technical and scientific support.
Funding
This work was supported by the Research Pro-Rectory of Federal University of Pará (PROPESP, UFPA, Brazil), Brazilian National Council for Scientific and Technological Development (CNPq), and Programa Nacional de Cooperação Acadêmica na Amazônia—PROCAD/Amazônia by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). This study was financed in part by the CAPES —Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures were reviewed and approved by the ethics committee on animal experimentation of the UFPA (protocol number 8613011217/CEUA-UFPA), according to the Guidelines for the Care and Use of Laboratory Animals [24]. Besides, this study followed the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guidelines (Table S1) [25].
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 858 kb).
Rights and permissions
About this article
Cite this article
Nascimento, P.C., Ferreira, M.M., Balbinot, K.M. et al. Methylmercury-Induced Toxicopathologic Findings in Salivary Glands of Offspring Rats After Gestational and Lactational Exposure. Biol Trace Elem Res 199, 2983–2991 (2021). https://doi.org/10.1007/s12011-020-02409-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02409-z