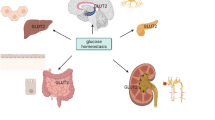
Abstract
Chromium (III) (Cr(III)) effect on improving glucose, body mass loss, and genomic stability has been extensively studied in models of type 2 diabetes. However, there is a lack of studies evaluating its effect on prediabetes. Thus, this study evaluates the effects of Cr(III) as dietetic supplementation on glucose metabolism, obesity, and genomic stability on prediabetic rat model using high-invert sugar. Male Wistar rats were divided randomly into four treatment groups: (1) control, receiving standard diet (control); (2) prediabetic (PD), receiving a 32% of invert sugar; (3) Cr(III), receiving chromium (III) chloride (CrCl3•6H2O) (58.4 mg/L); and (4) Cr(III) + PD, receiving CrCl3•6H2O in combination with high-invert sugar. Cr(III) supplementation significantly reduced blood glucose (123.00 ± 8.29 mg/dL vs. 115.30 ± 9.31 mg/dL, p = 0.015) and partially reduced area under the 120-min blood glucose response curve (AUC) in PD rats (p = 0.227). Moreover, Cr(III) attenuated weight gain (187.29 ± 38.56 g vs. 167.22 ± 29.30 g, p = 0.004), significantly reducing body mass index (0.68 ± 0.04 g/cm2 vs. 0.63 ± 0.04 g/cm2, p < 0.001), Lee index (0.30 ± 0.01 vs. 0.28 ± 0.01, p < 0.001), and peritoneal fat (p < 0.001). Regarding genomic stability, high-invert sugar, Cr(III), or the combination of both did not produce changes in oxidative stress, DNA damage in pancreas, or cytotoxicity markers. These data suggest that Cr(III) supplementation improved partially glucose metabolism and reduced obesity in rat model PD due to high-invert sugar without influence in genomic stability.


Similar content being viewed by others
Data Availability
Not applicable.
References
Siqueira JH, Mill JG, Velasquez-Melendez G, Moreira AD, Barreto SM, Benseñor IM, Molina MD (2018) Sugar-sweetened soft drinks and fructose consumption are associated with hyperuricemia: cross-sectional analysis from the Brazilian longitudinal study of adult health (ELSA-Brasil). Nutrients. 10:981. https://doi.org/10.3390/nu10080981
Mantantzis K, Schlaghecken F, Sünram-Lea SI, Maylor EA (2019) Sugar rush or sugar crash? A meta-analysis of carbohydrate effects on mood. Neurosci Biobehav Rev 101:45–67. https://doi.org/10.1016/j.neubiorev.2019.03.016
World Health Organization (2015) Guideline: sugars intake for adults and children. World Health Organization. https://www.who.int/publications/i/item/9789241549028. Accessed 8 June 2020
Imamura F, O’Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG (2016) Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ 50:496–504. https://doi.org/10.1136/bjsports-2016-h3576rep
Malik VS (2017) Sugar sweetened beverages and cardiometabolic health. Curr Opin Cardiol 32:572–579. https://doi.org/10.1161/CIRCULATIONAHA.109.876185
Drouin-Chartier JP, Zheng Y, Li Y, Malik V, Pan A, Bhupathiraju SN, Tobias DK, Manson JE, Willett WC, Hu FB (2019) Changes in consumption of sugary beverages and artificially sweetened beverages and subsequent risk of type 2 diabetes: results from three large prospective US cohorts of women and men. Diabetes Care 42:2181–2189. https://doi.org/10.2337/dc19-0734
American Diabetes Association (2020) 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care 43:S14. https://doi.org/10.2337/dc20-s002
Zaccardi F, Webb DR, Yates T, Davies MJ (2016) Pathophysiology of type 1 and type 2 diabetes mellitus: a 90-year perspective. Postgrad Med J 92:63–69. https://doi.org/10.1136/postgradmedj-2015-133281
Bigagli E, Lodovici M (2019) Circulating oxidative stress biomarkers in clinical studies on type 2 diabetes and its complications. Oxid Med Cell Longev. https://doi.org/10.1155/2019/5953685
Oguntibeju OO (2019) Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol 11:45–63
Ye X, Jiang R, Zhang Q, Wang R, Yang C, Ma J, Du H (2016) Increased 8-hydroxy-2′-deoxyguanosine in leukocyte DNA from patients with type 2 diabetes and microangiopathy. J Int Med Res 44:472–482. https://doi.org/10.1177/0300060515621530
Pereira CS, Molz P, Palazzo RP, de Freitas TA, Maluf SW, Horta JA, Prá D, Franke SI (2013) DNA damage and cytotoxicity in adult subjects with prediabetes. Mutat Res 753:76–81. https://doi.org/10.1016/j.mrgentox.2013.02.002
Ojeda JEO, Aguilar-Medina M, Olimón-Andalón V, Jau RAG, Ham AA, Quintana JGR, Silva-Benítez EL, Sanchez-Schmitz G, Ramos-Payán R (2018) Increased micronuclei frequency in oral and lingual epithelium of treated diabetes mellitus patients. Biomed Res Int 2018:1–8. https://doi.org/10.1155/2018/4898153
Marcovecchio ML (2017) Complications of acute and chronic hyperglycemia. US Endocrinol 13:01–17. https://doi.org/10.17925/USE.2017.13.01.17
Stehouwer CD (2018) Microvascular dysfunction and hyperglycemia: a vicious cycle with widespread consequences. Diabetes 67:1729–1741. https://doi.org/10.2337/dbi17-0044
Vincent JB (2017) New evidence against chromium as an essential trace element. J Nutr 147:2212–2219. https://doi.org/10.3945/jn.117.255901
Wise SS, Wise JP (2012) Chromium and genomic stability. Mutat Res 733:78–82. https://doi.org/10.1016/j.mrfmmm.2011.12.002
Vincent JB (2019) Effects of chromium supplementation on body composition, human and animal health, and insulin and glucose metabolism. Curr Opin Clin Nutr Metab Care 22:483–489. https://doi.org/10.1097/MCO.0000000000000604
Costello RB, Dwyer JT, Bailey RL (2016) Chromium supplements for glycemic control in type 2 diabetes: limited evidence of effectiveness. Nutr Rev 74:455–468. https://doi.org/10.1093/nutrit/nuw011
Huang H, Chen G, Dong Y, Zhu Y, Chen H (2018) Chromium supplementation for adjuvant treatment of type 2 diabetes mellitus: results from a pooled analysis. Mol Nutr Food Res 62:1700438. https://doi.org/10.1002/mnfr.201700438
Molz P, Molz WA, Dallemole DR, Santos LFS, Salvador M, Cruz DB, Pra D, Franke SIR (2020, 2020) Invert sugar induces glucose intolerance but does not cause injury to the pancreas nor permanent DNA damage in rats. An Acad Bras Cienc 92
McClain D (2002) Validation of models of cardiovascular disease in diabete. In: animal models of diabetic complications consortium protocols. Georgia Health Sciences University, Augusta https://www.diacomp.org/shared/document.aspx?id=11&docType=Protocol. Accessed 26 April 2020
Novelli EL, Diniz YS, Galhardi CM, Ebaid GM, Rodrigues HG, Mani F, Fernandes AA, Cicogna AC, Novelli Filho JL (2007) Anthropometrical parameters and markers of obesity in rats. Lab Anim 41:111–119. https://doi.org/10.1258/002367707779399518
Bernardis LL, Patterson BD (1968) Correlation between ‘Lee index’ and carcass fat content in weanling and adult female rats with hypothalamic lesions. J Endocrinol 40:527–528. https://doi.org/10.1677/joe.0.0400527
Molz P, Ellwanger JH, Zenkner FF, CAMPOS DD, Pra D, Putzke MT, Franke SI (2016) Recognition memory and DNA damage in undernourished young rats. An Acad Bras Cienc 88:1863–1873. https://doi.org/10.1590/0001-3765201620150608
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. https://doi.org/10.1016/0076-6879(90)86141-h
Grundy SM (2012) Pre-diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol 59:635–643. https://doi.org/10.1016/j.jacc.2011.08.080
Hafizur RM, Raza SA, Chishti S, Shaukat S, Ahmed AA (2015) Humanized’rat model of pre-diabetes by high fat diet-feeding to weaning wistar rats. Integr Obesity Diabetes 1:44–48. https://doi.org/10.15761/IOD.1000111
Al-Harbi MS, Hamza RZ (2016) Potential ameliorative effects of selenium and chromium supplementation against toxicity and oxidative stress in streptozotocin diabetic rats. Int J Pharmacol 12:483–495. https://doi.org/10.3923/ijp.2016.483.495
Doddigarla Z, Parwez I, Abidi S, Ahmad J (2016) Effect of chromium picolinate and melatonin either in single or in a combination in alloxan induced male Wistar rats. J Biomed Sci 6:1. https://doi.org/10.4172/2254-609X.100051
Sadri H, Larki NN, Kolahian S (2017) Hypoglycemic and hypolipidemic effects of leucine, zinc, and chromium, alone and in combination, in rats with type 2 diabetes. Biol Trace Elem Res 180:246–254. https://doi.org/10.1007/s12011-017-1014-2
Hanna HH, Rania H (2018) Effect of chromium piclonate versus L-carnitine on high fat diet induced in rats. Life Sci J 15. https://doi.org/10.7537/marslsj150618.02
Burgeiro A, Cerqueira MG, Varela-Rodríguez BM, Nunes S, Neto P, Pereira FC, Reis F, Carvalho E (2017) Glucose and lipid dysmetabolism in a rat model of prediabetes induced by a high-sucrose diet. Nutrients 9:638. https://doi.org/10.3390/nu9060638
Hostalek U (2019) Global epidemiology of prediabetes-present and future perspectives. Clin Diabetes Endocrinol 5:5. https://doi.org/10.1186/s40842-019-0080-0
American Diabetes Association (2014) Standards of medical care in diabetes-2014. Diabetes Care 37:S14–S80. https://doi.org/10.2337/dc14-S014
Vincent JB (2014) Is chromium pharmacologically relevant? J Trace Elem Med Biol 28:397–405. https://doi.org/10.1016/j.jtemb.2014.06.020
Nowotny K, Jung T, Höhn A, Weber D, Grune T (2015) Advanced glycation end products and oxidative stress in type 2 diabetes mellitus. Biomolecules 5:194–222. https://doi.org/10.3390/biom5010194
Franke SI, Molz P, Mai C, Ellwanger JH, Zenkner FF, Horta JA, Pra D (2017) High consumption of sucrose induces DNA damage in male Wistar rats. An Acad Bras Cienc 89:2657–2662. https://doi.org/10.1590/0001-3765201720160659
Al-Awar A, Kupai K, Veszelka M, Szűcs G, Attieh Z, Murlasits Z, Török S, Pósa A, Varga C (2016) Experimental diabetes mellitus in different animal models. J Diabetes Res 2016:1–12. https://doi.org/10.1155/2016/9051426
Maqbool M, Dar MA, Gani I, Mir SA (2019) Animal models in diabetes mellitus: an overview. J Drug Deliv Ther 9:472–475. https://doi.org/10.22270/jddt.v9i1-s.2351
Acknowledgments
The authors gratefully acknowledge the contribution of colleagues from the Laboratory of Histology and Pathology and Laboratory of Genetics and Biotechnology - UNISC for their help during the experiments.
Funding
PM was funded by the Foundation for Research Support of the State of Rio Grande do Sul (FAPERGS). SIRF was funded by the Brazilian National Council for Scientific and Technological Development (CNPq) grants.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethics Approval
The animals used in this study were processed in conformity with the ethical principles of animal experimentation, elaborated by the Brazilian College for Animal Experimentation (COBEA). The experimental procedures were approved by the Animal Ethics Committee of the University of Santa Cruz do Sul (Protocol n° 05/2011).
Consent to Participate
Not applicable.
Consent for Publication
All authors have approved the final version of this manuscript.
Code Availability
Not applicable.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Molz, P., Molz, W.A., Dallemole, D.R. et al. Potential Ameliorative Effects of Chromium Supplementation on Glucose Metabolism, Obesity, and Genomic Stability in Prediabetic Rat Model. Biol Trace Elem Res 199, 1893–1899 (2021). https://doi.org/10.1007/s12011-020-02299-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-020-02299-1