Abstract
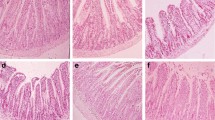
A total of 108 pregnant and lactation SD rats were divided into six groups, daily and orally dosed with Bacillus subtilis-copper (CuBs) and CuSO4 (CuS) both at three doses equivalent to 1.5 mg (Cu deficiency), 3 mg, and 6 mg Cu per kg diet, and the effects of the Cu source and dosage on the growth rate, immune status, duodenal development, and cecal microbial diversity were examined on 24-day-old offspring rats. The six offspring rats from each group were randomly selected for measuring the body weight gain and taking blood samples, and three rats were sacrificed for taking duodenum and cecum content samples. We found CuBs increased the body weight gain, development of duodenal villi, and survival rate of the offspring; increased the IgM content and lysozyme activity in serum; reduced the intestinal permeability; and increased the abundances of Lachnospiraceae, Ruminococcaceae, and Intestinibacter in the cecal content, when compared with CuS. We also found that Cu deficiency showed detrimental effects on the body weight gain and length, the survival rate of the offspring, and the immune indices in serum, as well as the increased intestinal permeability. We concluded that CuBs is better Cu source than CuSO4 for reproductive rats.



Similar content being viewed by others
References
Xia MS, Hu CH, Xu ZR (2005) Effects of copper bearing montmorillonite on the growth performance, intestinal microflora and morphology of weanling pigs. Anim Feed Sci Tech 118:307–317
Song J, Li YL, Hu CH (2013) Effects of copper-exchanged montmorillonite, as alternative to antibiotic, on diarrhea, intestinal permeability and proinflammatory cytokine of weanling pigs. Appl Clay Sci 77–78:52–55
Wang M, Du Y, Wang C, Tao W, He Y, Li H (2012) Effects of copper-loaded chitosan nanoparticles on intestinal microflora and morphology in weaned piglets. Biol Trace Elem Res 149:184–189
Liu D, Huang SM, Bi ZY, Wu C (2015) Optimization of zinc-riching conditions by probiotics in the form of mixing culture. J Anhui Agri Sci 43(20):259–262
Urrutia MM, Beveridge TJ (1994) Formation of fine-grained metal and silicate precipitates on a bacterial surface (Bacillus subtilis). Chem Geol 116:261–280
Magoc T, Salzberg SL (2011) Flash: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27:2957–2963
Pearson WR, Wood TC, Zhang Z, Miller W (1997) Comparison of DNA sequences with protein sequences. Genomics 46:24–36
Bolger A, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120
Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R (2011) Uchime improves sensitivity and speed of chimera detection. Bioinformatics 27:2194–2200
Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, Lesniewski RA, Oakley BB (2009) Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microb 75:7537–7541
Caporaso G, Stombaugh J, Jesse K, Knights D, Koenig JE, Ley RE, Lozupone CA, Mcdonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R (2010) Qiime allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microb 73:5261–5267
Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glockner FO (2012) The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:590–596
Zhang ZN, Wang BW, Ge WH, Zhang MA, Yue B, Zhang HW, Zhang YY (2016) Effects of Bacillus subtilis cooperate with copper on growth performance, slaughter performance, nutrient availability and meat quality of wulong geese aged from 5 to 16 weeks. Chinese J Anim Nutr 28:2830–2838
Wang Z, Cerrate S, Coto C, Yan F, Waldroup P (2007) Evaluation of MINTREX® copper as a source of copper in broiler diets. J Poult Sci 6:308–313
Zhao J, Shirley R, Vazquezanon M, Dibner J, Richards J (2010) Effects of chelated trace minerals on growth performance, breast meat yield, and footpad health in commercial meat broilers. J Appl Poult Res 19:365–372
Soetan K, Olaiya C, Oyewole O (2010) The importance of mineral elements for humans, domestic animals and plants - a review. African J Food Sci 4:200–222
Gaetke L, Chow CK (2003) Copper toxicity, oxidative stress, and antioxidant nutrients. Toxicology 189:147–163
Kim B, Nevitt T, Thiele D (2008) Mechanisms for copper acquisition, distribution and regulation. Nat Chem Biol 4:176–185
Creech B, Spears J, Flowers W, Hill G, Lloyd K (2004) Effect of dietary trace mineral concentration and source (inorganic vs. chelated) on performance, mineral status, and fecal mineral excretion in pigs from weaning through finishing. J Anim Sci 82:2140
Liao P, Li M, Li Y, Tan X, Zhao F, Shu X, Yin Y (2017) Effects of dietary supplementation with cupreous n-carbamylglutamate (NCG) chelate and copper sulfate on growth performance, serum biochemical profile and immune response, tissue mineral levels and fecal excretion of mineral in weaning piglets. Food Agri Immunol:1–15
Huang Y, Zhou T, Lee J, Jang H, Park J (2010) Effect of dietary copper sources (cupric sulfate and cupric methionate) and concentrations on performance and fecal characteristics in growing pigs. Asian-Aust J Ani Sci 23:757–761
Senthilkumar P, Nagalakshmi D, Reddy YR, Sudhakar K (2009) Effect of different level and source of copper supplementation on immune response and copper dependent enzyme activity in lambs. Trop Anim Health Pro 41:645–653
Molnar A, Podmaniczky B, Kurti P, Tenk I, Glavits R, Virag G, Szabo Z (2011) Effect of different concentrations of Bacillus subtilis on growth performance, carcase quality, gut microflora and immune response of broiler chickens. Brit Poultry Sci 52:658–665
Pabst O, Herbrand H, Friedrichsen M (2006) Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J Immunol 177:6824–6832
Prohaska J, Downing S, Lukasewycz O (1983) Chronic dietary copper deficiency alters biochemical and morphological properties of mouse lymphoid tissues. J Nutr 113:1583–1590
Bae B, Percival SS (1993) Retinoic acid-induced hl-60 cell differentiation is augmented by copper supplementation. J Nutr 123:997–1002
Faries P, Simon R, Martella A, Lee M, Machiedo G (1999) Intestinal permeability correlates with severity of injury in trauma patients. Surg Clin N Am 79:1373–1383
Ng SC, Hart A, Kamm MA, Stagg AJ, Knight SC (2009) Mechanisms of action of probiotics: recent advances. Inflamm Bowel Dis 15:300–310
Linder MC, Hazeghazam M (1996) Copper biochemistry and molecular biology. Am J Clin Nutr 63:797–811
Tomaszewska E, Dobrowolski P, Kwiecien M (2016) Intestinal alterations, basal hematology, and biochemical parameters in adolescent rats fed different sources of dietary copper. Biol Trace Elem Res 171:185–191
Yirga H (2015) The use of probiotics in animal nutrition. J Prob & Health 3:1–10
Cho JH, Zhao PY, Kim IH (2011) Probiotics as a dietary additive for pigs: a review. J Anim Vet Adv 10:2127–2134
Hsieh YY, Tung SY, Pan HY, Yen CW, Xu HW, Lin YJ, Deng YF, Hsu WT, Wu CS, Li C (2008) Increased abundance of clostridium and fusobacterium in gastric microbiota of patients with gastric cancer in Taiwan. Sci Rep 8:158–169
Li YY, Ge QX, Cao J, Zhou YJ, Du YL, Shen B, Wan YJ, Nie YQ (2016) Association of fusobacterium nucleatum infection with colorectal cancer in Chinese patients. World J Gastroentero 22:3227–3233
Fukugaiti MH, Ignacio A, Fernandes MR, Ulysses Ribeiro J, Nakano V, Avila-Campos MJ (2015) High occurrence of fusobacterium nucleatum and clostridium difficile in the intestinal microbiota of colorectal carcinoma patients. Braz J Microbiol 46:1135–1140
Ohman L, Tornblom H, Simren M (2015) Crosstalk at the mucosal border: importance of the gut microenvironment in IBS. Nat Rev Gastro Hepat 12:36–49
Biddle A, Stewart L, Blanchard J, Leschine S (2013) Untangling the genetic basis of fibrolytic specialization by lachnospiraceae and ruminococcaceae in diverse gut communities. Diversity 5:627–640
Louis P, Flint HJ (2009) Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol Lett 294:1–8
Huws S, Kim E, Lee M, Scott M, Tweed J, Pinloche E, Wallace R, Scollan N (2011) As yet uncultured bacteria phylogenetically classified as Prevotella, Lachnospiraceae incertae sedis and unclassified Bacteroidales, Clostridiales and Ruminococcaceae may play a predominant role in ruminal biohydrogenation. Environ Microbiol 13:1500–1512
Haberle J, Gorg B, Rutsch F, Schmidt E, Toutain A, Benoist J, Gelot A, Suc A, Hohne W, Schliess F, Haussinger D, Koch HG (2005) Congenital glutamine deficiency with glutamine synthetase mutations. New Engl J Med 353:1926–1933
Yirga H (2015) The use of probiotics in animal nutrition. Journal of Probiotics & Health 3:1–10
Parvez S, Malik KA, Kang A, Kim HY (2006) Probiotics and their fermented food products are beneficial for health. J Appl Microbiol 100:1171–1185
Patterson JA, Burkholder KM (2003) Application of prebiotics and probiotics in poultry production. Poultry Sci 82:627–631
Lei X, Piao X, Ru Y, Zhang H, Péron A, Zhang H (2015) Effect of bacillus amyloliquefaciens-based direct-fed microbial on performance, nutrient utilization, intestinal morphology and cecal microflora in broiler chickens. Asian-Aust J Anim Sci 28:239–246
Lescheid DW (2014) Probiotics as regulators of inflammation: a review. J Funct Foods 4:299–311
Funding
This study is supported by the grants from the National Waterfowl Industry Technology System Foundation of China (No. CARS-43-11) and the National Key R&D Program “Application and Demonstration of Green Waterfowl Efficient Safe Aquaculture Technology” (2018YFD0501501).
Author information
Authors and Affiliations
Contributions
G.L., B.W., W.G., and M.Z. conceived and designed the experiments; G.L., W.G., and B.Y. performed the experiments; G.L. and Y.H. analyzed the data; and G.L., K.L., and M.K. wrote the paper. All of the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
This study was approved by the Animal Ethics Committee of the College of Animal Science and Technology, Qingdao Agricultural University, China.
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, G., Wang, B., Huang, Y. et al. The Growth Rate, Immune Status, Duodenal Development, and Cecal Microbial Diversity of 24-Day-Old Offspring of SD Rats Received Bacillus subtilis-Cu or CuSO4 During Pregnancy and Lactation Periods. Biol Trace Elem Res 191, 435–442 (2019). https://doi.org/10.1007/s12011-019-1638-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-1638-5