Abstract
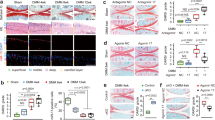
Osteoarthritis (OA), a common degenerative disease affecting articular cartilage, is caused by multiple factors, and currently, there are few approaches to effectively delay its progression. This study aimed to evaluate whether a strontium compound (in the form of strontium gluconate, Glu-Sr) could reduce OA pathology severity in osteoarthritic rat models by directly targeting chondrocytes, including catabolic/anabolic activities and/or chondrogenic differentiation. Glu-Sr was administered to OA rats by oral gavage beginning during OA induction and continuing for 8 weeks. Glu-Sr treatment was found to significantly reduce cartilage degeneration and delay OA progression. Further examination showed that collagen II, Sox9, and aggrecan (ACAN) genes were up-regulated whereas IL-1β was down-regulated in chondrocytes isolated from Glu-Sr-treated rats. Glu-Sr also antagonized the catabolic effects of IL-1β on chondrocytes. Furthermore, Glu-Sr was shown to promote the chondrogenic differentiation of bone marrow mesenchymal stem cells (BMMSCs), possibly through promoting chondrogenic gene expression, including CTGF and FGF1, as revealed by RNA-sequencing (RNA-seq). These results suggest that systemic administration of Glu-Sr may be useful in prophylactic and therapeutic treatment of chronic cartilage degradation through affecting multiple steps from chondrogenic differentiation of progenitors to matrix formation in mature chondrocytes.





Similar content being viewed by others
References
Lane NE, Brandt K, Hawker G, Peeva E, Schreyer E, Tsuji W, Hochberg MC (2011) OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthr Cartil 19(5):478–482. https://doi.org/10.1016/j.joca.2010.09.013
Hochberg MC, Altman RD, Karine Toupin A, Maria B, Gordon G, Jessie MG, Tanveer T, Vivian W, George W, Peter T (2012) American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthrit Care Res 41(1):92–93
Hunter DJ, Felson DT (2006) Osteoarthritis. BMJ 332(7542):639–642. https://doi.org/10.1136/bmj.332.7542.639
Smelter E, Hochberg MC (2013) New treatments for osteoarthritis. Curr Opin Rheumatol 25(3):310–316. https://doi.org/10.1097/BOR.0b013e32835f69b4
Kaufman JM, Audran M, Bianchi G, Braga V, Diaz-Curiel M, Francis RM, Goemaere S, Josse R, Palacios S, Ringe JD, Felsenberg D, Boonen S (2013) Efficacy and safety of strontium ranelate in the treatment of osteoporosis in men. J Clin Endocrinol Metab 98(2):592–601. https://doi.org/10.1210/jc.2012-3048
Saffar JL (2004) The effects of strontium Ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350(5):459–468
Tan S, Zhang B, Zhu X, Ao P, Guo H, Yi W, Zhou GQ (2014) Deregulation of bone forming cells in bone diseases and anabolic effects of strontium-containing agents and biomaterials. Biomed Res Int 2014:814057. https://doi.org/10.1155/2014/814057
Yuan R, Ma S, Zhu X, Li J, Liang Y, Liu T, Zhu Y, Zhang B, Tan S, Guo H (2016) Core level regulatory network of osteoblast as molecular mechanism for osteoporosis and treatment. Oncotarget 7(4):3692–3701
Fonseca JE (2008) Rebalancing bone turnover in favour of formation with strontium ranelate: implications for bone strength. Rheumatology 47(Suppl 4):iv17–iv19
PJ M (2006) Strontium ranelate: a dual mode of action rebalancing bone turnover in favour of bone formation. Curr Opin Rheumatol 18(Suppl 1):S11
Han W, Fan S, Bai X, Ding C (2017) Strontium Ranelate, a promising disease modifying osteoarthritis drug. Expert Opin Investig Drugs 26(3):375–380
Tenti S, Cheleschi S, Guidelli GM, Galeazzi M, Fioravanti A (2014) What about strontium ranelate in osteoarthritis? Doubts and securities. Mod Rheumatol 24(6):881–884. https://doi.org/10.3109/14397595.2014.888156
Yu DG, Ding HF, Mao YQ, Liu M, Yu B, Zhao X, Wang XQ, Li Y, Liu GW, Nie SB, Liu S, Zhu ZA (2013) Strontium ranelate reduces cartilage degeneration and subchondral bone remodeling in rat osteoarthritis model. Acta Pharmacol Sin 34(3):393–402. https://doi.org/10.1038/aps.2012.167
Pelletier JP, Kapoor M, Fahmi H, Lajeunesse D, Blesius A, Maillet J, Martel-Pelletier J (2013) Strontium ranelate reduces the progression of experimental dog osteoarthritis by inhibiting the expression of key proteases in cartilage and of IL-1beta in the synovium. Ann Rheum Dis 72(2):250–257. https://doi.org/10.1136/annrheumdis-2012-201710
Bruyère O, Reginster J-Y, Bellamy N, Chapurlat R, Richette P, Cooper C, on behalf of the Si (2014) Clinically meaningful effect of strontium ranelate on symptoms in knee osteoarthritis: a responder analysis. Rheumatology 53(8):1457–1464. https://doi.org/10.1093/rheumatology/keu018
Alexandersen P, Karsdal MA, Byrjalsen I, Christiansen C (2011) Strontium ranelate effect in postmenopausal women with different clinical levels of osteoarthritis. Climacteric 14(2):236–243
Pelletier J-P, Roubille C, Raynauld J-P, Abram F, Dorais M, Delorme P, Martel-Pelletier J (2015) Disease-modifying effect of strontium ranelate in a subset of patients from the phase III knee osteoarthritis study SEKOIA using quantitative MRI: reduction in bone marrow lesions protects against cartilage loss. Ann Rheum Dis 74(2):422–429. https://doi.org/10.1136/annrheumdis-2013-203989
Cyrus C, Jean-Yves R, Roland C, Claus C, Harry G, Nicholas B, William B, Federico N, Janusz B, Evgeny N (2012) Efficacy and safety of oral strontium ranelate for the treatment of knee osteoarthritis: rationale and design of randomised, double-blind, placebo-controlled trial. Curr Med Res Opin 28(2):231–239
Bruyere O, Delferriere D, Roux C, Wark JD, Spector T, Devogelaer JP, Brixen K, Adami S, Fechtenbaum J, Kolta S, Reginster JY (2008) Effects of strontium ranelate on spinal osteoarthritis progression. Ann Rheum Dis 67(3):335–339. https://doi.org/10.1136/ard.2007.075572
Kuhn K, D'Lima DD, Hashimoto S, Lotz M (2004) Cell death in cartilage. Osteoarthr Cartil 12(1):1–16
Maldonado M, Nam J (2013) The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res Int 2013:284873. https://doi.org/10.1155/2013/284873
Henrotin Y, Labasse A, Zheng SX, Galais P, Tsouderos Y, Crielaard JM, Reginster JY (2010) Strontium Ranelate increases cartilage matrix formation. J Bone Miner Res 16(2):299–308
Iijima H, Aoyama T, Ito A, Tajino J, Nagai M, Zhang X, Yamaguchi S, Akiyama H, Kuroki H (2014) Destabilization of the medial meniscus leads to subchondral bone defects and site-specific cartilage degeneration in an experimental rat model. Osteoarthr Cartil 22(7):1036–1043
Iijima H, Aoyama T, Ito A, Yamaguchi S, Nagai M, Tajino J, Zhang X, Kuroki H (2015) Effects of short-term gentle treadmill walking on subchondral bone in a rat model of instability-induced osteoarthritis. Osteoarthr Cartil 23(9):1563–1574
Rim YA, Nam Y, Park N, Jung H, Jang Y, Lee J, Ju JH (2018) Different Chondrogenic potential among human induced pluripotent stem cells from diverse origin primary cells. Stem Cells Int 2018(3):1–13
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45
Toh WS, Lee EH, Richards M, Cao T (2010) In vitro derivation of chondrogenic cells from human embryonic stem cells. Methods Mol Biol 584:317–331. https://doi.org/10.1007/978-1-60761-369-5_17
Pritzker KP, Gay S, Jimenez SA, Ostergaard K, Pelletier JP, Revell PA, Salter D, van den Berg WB (2006) Osteoarthritis cartilage histopathology: grading and staging. Osteoarthr Cartil 14(1):13–29. https://doi.org/10.1016/j.joca.2005.07.014
Yao L, Xue X, Yu P, Ni Y, Chen F (2018) Evans blue dye: a revisit of its applications in biomedicine. Contrast Media Mol Imaging 2018:7628037. https://doi.org/10.1155/2018/7628037
de Melo Nunes R, Martins MR, da Silva Junior FS, de Melo Leite AC, Girao VC, de Queiroz Cunha F, Marinho AL, Pinto AC, Rocha FA (2015) Strontium ranelate analgesia in arthritis models is associated to decreased cytokine release and opioid-dependent mechanisms. Inflamm Res 64(10):781–787. https://doi.org/10.1007/s00011-015-0860-7
Mierzwa AG, Campos JF, Jesus MF, Nader HB, Lazaretticastro M, Reginato RD (2017) Different doses of strontium ranelate and mechanical vibration modulate distinct responses in the articular cartilage of ovariectomized rats. Osteoarthr Cartil 25:1179–1188
Strontium ranelate discontinued (2017). Drug Ther Bull 55 (8):93–94. https://doi.org/10.1136/dtb.2017.8.0516
Makki M, Haqqi T (2017) Histone deacetylase inhibitor vorinostat (SAHA, MK0683) perturb miR-9-MCPIP1 axis to block IL-1β-induced IL-6 expression in human OA chondrocytes. Connect Tissue Res 58(1):64–75
Liu YD, Yang HX, Liao LF, Jiao K, Zhang HY, Lu L, Zhang M, Zhang J, He JJ, Wu YP, Chen D, Wang MQ (2016) Systemic administration of strontium or NBD peptide ameliorates early stage cartilage degradation of mouse mandibular condyles. Osteoarthr Cartil 24(1):178–187. https://doi.org/10.1016/j.joca.2015.07.022
Okita N, Honda Y, Kishimoto N, Liao W, Azumi E, Hashimoto Y, Matsumoto N (2015) Supplementation of strontium to a chondrogenic medium promotes chondrogenic differentiation of human dedifferentiated fat cells. Tissue Eng Part A 21(9–10):1695–1704. https://doi.org/10.1089/ten.TEA.2014.0282
Acknowledgments
The authors would like to thank Novogene Company (Beijing, China) for providing some of the RNA-seq files whose data were used in this study, and Chemical and Biological Center of Shenzhen University for providing Strontium gluconate.
Funding
This work was funded by the National Natural Science Foundation of China (81472126), Shenzhen Scientific and Innovative Commission (JCYJ20160226192924528, JCYJ20160331114205502 and GGFW2016030117123665), and the Shenzhen Development and Reform Committee for Shenzhen Engineering Laboratory of Orthopedic Regenerative Technologies.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
The protocol for animal care and use conformed to the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Hu, P., Du, J., Zhang, S. et al. Oral Administration of Strontium Gluconate Effectively Reduces Articular Cartilage Degeneration Through Enhanced Anabolic Activity of Chondrocytes and Chondrogenetic Differentiation of Mesenchymal Stromal Cells. Biol Trace Elem Res 193, 422–433 (2020). https://doi.org/10.1007/s12011-019-01711-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01711-9