Abstract
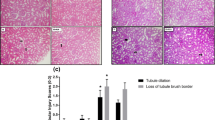
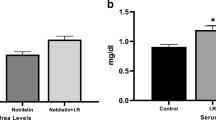
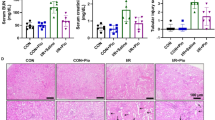
The purpose of this research was to evaluate the protective effects of apocynin on renal ischemia/reperfusion (I/R) injury (RI/RI) in rats. Rats preconditioned with apocynin were subjected to renal I/R. Zinc levels in serum and renal tissues, blood urea nitrogen (BUN), and serum creatinine (Scr) were detected. We further measured the activity of superoxide dismutase (SOD); the content of malondialdehyde (MDA), IL-4, IL-6, IL-10, and TNF-α; and the expression of metallothionein (MT) in the renal tissues. Results indicated that the levels of MDA, IL-4, IL-6, IL-10, TNF-α, and MT in the kidney tissue and serum BUN and Scr levels in RI/RI group were significantly higher than those in sham-operated group, while the levels of serum Zn and kidney Zn and SOD were reduced in RI/RI group. Apocynin treatment further decreased the levels of MDA, IL-6, TNF-α, and serum BUN and Scr, whereas it significantly increased the levels of Zn, SOD, IL-4, IL-10, and MT in the kidney tissue and serum Zn. These findings suggest that apocynin might play a protective role against RI/RI in rats through regulating zinc level and MT expression involving in oxidative stress.





Similar content being viewed by others
References
Carden DL, Granger DN (2000) Pathophysiology of ischaemia-reperfusion injury. J Pathol 190(3):255–266. doi:10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6
Zhou SP, Liao WT, Yang LK, Sun L (2013) Effects of sevoflurane pretreatment on renal Src and FAK expression in diabetic rats after renal ischemia/reperfusion injury. Mol Cell Biochem 384(1–2):203–211. doi:10.1007/s11010-013-1799-z
Sun N, Wang H, Wang L (2016) Protective effects of ghrelin against oxidative stress, inducible nitric oxide synthase and inflammation in a mouse model of myocardial ischemia/reperfusion injury via the HMGB1 and TLR4/NF-kappaB pathway. Mol Med Rep 14(3):2764–2770. doi:10.3892/mmr.2016.5535
Chen YY, Yeh CH, So EC, Sun DP, Wang LY, Hsing CH (2014) Anticancer drug 2-methoxyestradiol protects against renal ischemia/reperfusion injury by reducing inflammatory cytokines expression. Biomed Res Int 2014:431524. doi:10.1155/2014/431524
Hasan R, Rink L, Haase H (2016) Chelation of free Zn(2)(+) impairs chemotaxis, phagocytosis, oxidative burst, degranulation, and cytokine production by neutrophil granulocytes. Biol Trace Elem Res 171(1):79–88. doi:10.1007/s12011-015-0515-0
Cortese-Krott MM, Kulakov L, Oplander C, Kolb-Bachofen V, Kroncke KD, Suschek CV (2014) Zinc regulates iNOS-derived nitric oxide formation in endothelial cells. Redox Biol 2:945–954. doi:10.1016/j.redox.2014.06.011
Xu G, Hu B, Chen G, Yu X, Luo J, Lv J, Gu J (2015) Analysis of blood trace elements and biochemical indexes levels in severe craniocerebral trauma adults with Glasgow coma scale and injury severity score. Biol Trace Elem Res 164(2):192–197. doi:10.1007/s12011-014-0225-z
Kambe T, Tsuji T, Hashimoto A, Itsumura N (2015) The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol Rev 95(3):749–784. doi:10.1152/physrev.00035.2014
Kimura T, Kambe T (2016) The functions of metallothionein and ZIP and ZnT transporters: an overview and perspective. Int J Mol Sci 17(3):336. doi:10.3390/ijms17030336
Billur D, Tuncay E, Okatan EN, Olgar Y, Durak AT, Degirmenci S, Can B, Turan B (2016) Interplay between cytosolic free Zn2+ and mitochondrion morphological changes in rat ventricular cardiomyocytes. Biol Trace Elem Res. doi:10.1007/s12011-016-0704-5
Felizola SJ, Nakamura Y, Arata Y, Ise K, Satoh F, Rainey WE, Midorikawa S, Suzuki S, Sasano H (2014) Metallothionein-3 (MT-3) in the human adrenal cortex and its disorders. Endocr Pathol 25(3):229–235. doi:10.1007/s12022-013-9280-9
Costello LC, Fenselau CC, Franklin RB (2011) Evidence for operation of the direct zinc ligand exchange mechanism for trafficking, transport, and reactivity of zinc in mammalian cells. J Inorg Biochem 105(5):589–599. doi:10.1016/j.jinorgbio.2011.02.002
Fukada T, Kambe T (2011) Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 3(7):662–674. doi:10.1039/c1mt00011j
Knoch ME, Hartnett KA, Hara H, Kandler K, Aizenman E (2008) Microglia induce neurotoxicity via intraneuronal Zn(2+) release and a K(+) current surge. Glia 56(1):89–96. doi:10.1002/glia.20592
Tuncay E, Turan B (2016) Intracellular Zn(2+) increase in cardiomyocytes induces both electrical and mechanical dysfunction in heart via endogenous generation of reactive nitrogen species. Biol Trace Elem Res 169(2):294–302. doi:10.1007/s12011-015-0423-3
Wang G, Huang H, Zheng H, He Y, Zhang Y, Xu Z, Zhang L, Xi J (2016) Zn2+ and mPTP mediate endoplasmic reticulum stress inhibition-induced cardioprotection against myocardial ischemia/reperfusion injury. Biol Trace Elem Res 174(1):189–197. doi:10.1007/s12011-016-0707-2
Slepchenko KG, Lu Q, Li YV (2016) Zinc wave during the treatment of hypoxia is required for initial reactive oxygen species activation in mitochondria. Int J Physiol Pathophysiol Pharmacol 8(1):44–51
Choi EK, Jung H, Kwak KH, Yeo J, Yi SJ, Park CY, Ryu TH, Jeon YH, Park KM, Lim DG (2015) Effects of allopurinol and apocynin on renal ischemia-reperfusion injury in rats. Transplant Proc 47(6):1633–1638. doi:10.1016/j.transproceed.2015.06.007
Ozbek O, Altintas R, Polat A, Vardi N, Parlakpinar H, Sagir M, Duran ZR, Yildiz A (2015) The protective effect of apocynin on testicular ischemia-reperfusion injury. J Urol 193(4):1417–1422. doi:10.1016/j.juro.2014.11.086
Romanini CV, Ferreira ED, Soares LM, Santiago AN, Milani H, de Oliveira RM (2015) 4-hydroxy-3-methoxy-acetophenone-mediated long-lasting memory recovery, hippocampal neuroprotection, and reduction of glial cell activation after transient global cerebral ischemia in rats. J Neurosci Res 93(8):1240–1249. doi:10.1002/jnr.23575
Sener TE, Yuksel M, Ozyilmaz-Yay N, Ercan F, Akbal C, Simsek F, Sener G (2015) Apocynin attenuates testicular ischemia-reperfusion injury in rats. J Pediatr Surg 50(8):1382–1387. doi:10.1016/j.jpedsurg.2014.11.033
Uysal A, Sahna E, Ozguler IM, Burma O, Ilhan N (2015) Effects of apocynin, an NADPH oxidase inhibitor, on levels of ADMA, MPO, iNOS and TLR4 induced by myocardial ischemia reperfusion. Perfusion 30(6):472–477. doi:10.1177/0267659114559260
Kimura K, Shirabe K, Yoshizumi T, Takeishi K, Itoh S, Harimoto N, Ikegami T, Uchiyama H, Okano S, Maehara Y (2016) Ischemia-reperfusion injury in fatty liver is mediated by activated NADPH oxidase 2 in rats. Transplantation 100(4):791–800. doi:10.1097/TP.0000000000001130
Li Z, Wang Y (2015) Effect of NADPH oxidase inhibitor-apocynin on the expression of Src homology-2 domain-containing phosphatase-1 (SHP-1) exposed renal ischemia/reperfusion injury in rats. Toxicology Reports 2:1111–1116. doi:10.1016/j.toxrep.2015.07.019
Tang LL, Ye K, Yang XF, Zheng JS (2007) Apocynin attenuates cerebral infarction after transient focal ischaemia in rats. J Int Med Res 35(4):517–522
Song SX, Gao JL, Wang KJ, Li R, Tian YX, Wei JQ, Cui JZ (2013) Attenuation of brain edema and spatial learning de fi cits by the inhibition of NADPH oxidase activity using apocynin following diffuse traumatic brain injury in rats. Mol Med Rep 7(1):327–331. doi:10.3892/mmr.2012.1147
Xu G, Su R, Li B, Lv J, Sun W, Hu B, Li X, Gu J, Yu X (2015) Trace element concentrations in human tissues of death cases associated with secondary infection and MOF after severe trauma. Biol Trace Elem Res 168(2):335–339. doi:10.1007/s12011-015-0378-4
Tong F, Luo L, Liu DJ (2016) Effect of intervention in mast cell function before reperfusion on renal ischemia-reperfusion injury in rats. Kidney Blood Press Res 41(3):335–344. doi:10.1159/000443437
Tong F, Tang X, Li X, Xia W, Liu D (2016) The effect of insulin-loaded linear poly(ethylene glycol)-brush-like poly(L-lysine) block copolymer on renal ischemia/reperfusion-induced lung injury through downregulating hypoxia-inducible factor. Int J Nanomedicine 11:1717–1730. doi:10.2147/Ijn.S99890
Jiang Y, Zhou Z, Meng QT, Sun Q, Su W, Lei S, Xia Z, Xia ZY (2015) Ginsenoside Rb1 treatment attenuates pulmonary inflammatory cytokine release and tissue injury following intestinal ischemia reperfusion injury in mice. Oxidative Med Cell Longev 2015:843721. doi:10.1155/2015/843721
Lopert P, Day BJ, Patel M (2012) Thioredoxin reductase deficiency potentiates oxidative stress, mitochondrial dysfunction and cell death in dopaminergic cells. PLoS One 7(11) ARTN e50683. doi:10.1371/journal.pone.0050683
Campbell L, Howie F, Arthur JR, Nicol F, Beckett G (2007) Selenium and sulforaphane modify the expression of selenoenzymes in the human endothelial cell line EAhy926 and protect cells from oxidative damage. Nutrition 23(2):138–144. doi:10.1016/j.nut.2006.10.006
Maldonado PD, Perez-De La Cruz V, Torres-Ramos M, Silva-Islas C, Lecona-Vargas R, Lugo-Huitron R, Blanco-Ayala T, Ugalde-Muniz P, Vazquez-Cervantes GI, Fortoul TI, Ali SF, Santamaria A (2012) Selenium-induced antioxidant protection recruits modulation of thioredoxin reductase during excitotoxic/pro-oxidant events in the rat striatum. Neurochem Int 61(2):195–206. doi:10.1016/j.neuint.2012.05.004
Pan R, Timmins GS, Liu W, Liu KJ (2015) Autophagy mediates astrocyte death during zinc-potentiated ischemia-reperfusion injury. Biol Trace Elem Res 166(1):89–95. doi:10.1007/s12011-015-0287-6
Gholami M, Zendedel A, Khanipour khayat Z, Ghanad K, Nazari A, Pirhadi A (2015) Selenium effect on ischemia-reperfusion injury of gastrocnemius muscle in adult rats. Biol Trace Elem Res 164(2):205–211. doi:10.1007/s12011-014-0218-y
Bernardi A, Frozza RL, Hoppe JB, Salbego C, Pohlmann AR, Battastini AM, Guterres SS (2013) The antiproliferative effect of indomethacin-loaded lipid-core nanocapsules in glioma cells is mediated by cell cycle regulation, differentiation, and the inhibition of survival pathways. Int J Nanomedicine 8:711–728. doi:10.2147/IJN.S40284
Lv M, Fu X, Hu L, Yue X, Han X (2016) The expression of zinc transporters changed in the intestine of weaned pigs exposed to zinc chitosan chelate. Biol Trace Elem Res. doi:10.1007/s12011-016-0732-1
Brandt EG, Hellgren M, Brinck T, Bergman T, Edholm O (2009) Molecular dynamics study of zinc binding to cysteines in a peptide mimic of the alcohol dehydrogenase structural zinc site. Phys Chem Chem Phys 11(6):975–983. doi:10.1039/b815482a
Plum LM, Rink L, Haase H (2010) The essential toxin: impact of zinc on human health. Int J Environ Res Public Health 7(4):1342–1365. doi:10.3390/ijerph7041342
Ganger R, Garla R, Mohanty BP, Bansal MP, Garg ML (2016) Protective effects of zinc against acute arsenic toxicity by regulating antioxidant defense system and cumulative metallothionein expression. Biol Trace Elem Res 169(2):218–229. doi:10.1007/s12011-015-0400-x
Ooi TC, Chan KM, Sharif R (2016) Zinc carnosine inhibits lipopolysaccharide-induced inflammatory mediators by suppressing NF-kappab activation in raw 264.7 macrophages, independent of the MAPKs signaling pathway. Biol Trace Elem Res 172(2):458–464. doi:10.1007/s12011-015-0615-x
Skalny AA, Tinkov AA, Medvedeva YS, Alchinova IB, Karganov MY, Ajsuvakova OP, Skalny AV, Nikonorov AA (2015) Zinc asparaginate supplementation induces redistribution of toxic trace elements in rat tissues and organs. Interdiscip Toxicol 8(3):131–138. doi:10.1515/intox-2015-0020
Ribeiro SM, Braga CB, Peria FM, Domenici FA, Martinez EZ, Feres O, da Rocha JJ, da Cunha SF (2016) Effect of zinc supplementation on antioxidant defenses and oxidative stress markers in patients undergoing chemotherapy for colorectal cancer: a placebo-controlled, prospective randomized trial. Biol Trace Elem Res 169(1):8–16. doi:10.1007/s12011-015-0396-2
Maret W (2011) Redox biochemistry of mammalian metallothioneins. J Biol Inorg Chem 16(7):1079–1086. doi:10.1007/s00775-011-0800-0
Lazo JS, Kondo Y, Dellapiazza D, Michalska AE, Choo KH, Pitt BR (1995) Enhanced sensitivity to oxidative stress in cultured embryonic cells from transgenic mice deficient in metallothionein I and II genes. J Biol Chem 270(10):5506–5510
Wu H, Kong L, Cheng Y, Zhang Z, Wang Y, Luo M, Tan Y, Chen X, Miao L, Cai L (2015) Metallothionein plays a prominent role in the prevention of diabetic nephropathy by sulforaphane via up-regulation of Nrf2. Free Radic Biol Med 89:431–442. doi:10.1016/j.freeradbiomed.2015.08.009
Leierer J, Rudnicki M, Braniff SJ, Perco P, Koppelstaetter C, Muhlberger I, Eder S, Kerschbaum J, Schwarzer C, Schroll A, Weiss G, Schneeberger S, Wagner S, Konigsrainer A, Bohmig GA, Mayer G (2016) Metallothioneins and renal ageing. Nephrol Dial Transplant 31(9):1444–1452. doi:10.1093/ndt/gfv451
Palmiter RD (1995) Constitutive expression of metallothionein-III (MT-III), but not MT-I, inhibits growth when cells become zinc deficient. Toxicol Appl Pharmacol 135(1):139–146. doi:10.1006/taap.1995.1216
Heumuller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schroder K, Brandes RP (2008) Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension 51(2):211–217. doi:10.1161/HYPERTENSIONAHA.107.100214
Stefanska J, Sarniak A, Wlodarczyk A, Sokolowska M, Pniewska E, Doniec Z, Nowak D, Pawliczak R (2012) Apocynin reduces reactive oxygen species concentrations in exhaled breath condensate in asthmatics. Exp Lung Res 38(2):90–99. doi:10.3109/01902148.2011.649823
Altintas R, Polat A, Vardi N, Oguz F, Beytur A, Sagir M, Yildiz A, Parlakpinar H (2013) The protective effects of apocynin on kidney damage caused by renal ischemia/reperfusion. J Endourol 27(5):617–624. doi:10.1089/end.2012.0556
Acknowledgements
The authors appreciate the support from the financial support from the Science and Technology Planning Project of Zhejiang Province (2015C37130), the Zhejiang Provincial Natural Science Foundation (LQ14H230001), and the Science and Technology Planning Project of Jiaxing (2012AY1072-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Bo Hu and Yuhong Wu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Hu, B., Wu, Y., Tong, F. et al. Apocynin Alleviates Renal Ischemia/Reperfusion Injury Through Regulating the Level of Zinc and Metallothionen. Biol Trace Elem Res 178, 71–78 (2017). https://doi.org/10.1007/s12011-016-0904-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0904-z