Abstract
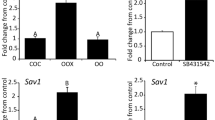
Progesterone production is upregulated in granulosa cells (cumulus and mural) after the LH surge, but the intra-follicular mechanisms regulating this transition are not completely known. Recent findings show that the transition metal chelator, N,N,N′,N′-tetrakis-(2-pyridylmethyl)-ethylenediamine (TPEN), impairs ovarian function. In this study, we provide evidence that chelating transition metals, including zinc, enhances progesterone production. The findings show that TPEN (transition metal chelator) increases abundance of Cyp11a1 and Star messenger RNA (mRNA) between 8- and 20-fold and progesterone production more than 3-fold in cultured cumulus-oocyte complexes (COC). Feeding a zinc-deficient diet for 10 days, but not 3 days, increased Star, Hsd3b, and prostaglandin F2 alpha receptor (Ptgfr) mRNA ~2.5-fold, suggesting that the effect of TPEN is through modulation of zinc availability. Progesterone from cumulus cells promotes oocyte developmental potential. Blocking progesterone production with epostane during maturation reduced subsequent blastocyst formation from 89 % in control to 18 % in epostane-treated complexes, but supplementation with progesterone restored blastocyst developmental potential to 94 %. Feeding a zinc-deficient diet for 5 days before ovulation did not affect the number of CL, STAR protein, or serum progesterone. However, incubating luteal tissue with TPEN increased abundance of Star, Hsd3b, and Ptgfr mRNA 2–3-fold and increased progesterone production 3-fold. TPEN is known to abolish SMAD2/3 signaling in cumulus cells. However, treatment of COC with the SMAD2/3 phosphorylation inhibitor, SB421542, did not by itself induce steroidogenic transcripts but did potentiate EGF-induced Star mRNA expression. Collectively, the results show that depletion of transition metals with TPEN acutely enhances progesterone biosynthesis in COC and luteal tissue.






Similar content being viewed by others
References
Park JY, YQ S, Ariga M, Law E, Jin SL, Conti M (2004) EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science 303(5658):682–684
Hsieh M, Lee D, Panigone S, Horner K, Chen R, Theologis A, Lee DC, Threadgill DW, Conti M (2007) Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol 27(5):1914–1924. doi:10.1128/mcb.01919-06
Y-Q S, Nyegaard M, Overgaard MT, Qiao J, Giudice LC (2006) Participation of mitogen-activated protein kinase in luteinizing hormone-induced differential regulation of steroidogenesis and steroidogenic gene expression in mural and cumulus granulosa cells of mouse preovulatory follicles. Biol Reprod 75(6):859–867. doi:10.1095/biolreprod.106.052613
Panigone S, Hsieh M, Fu M, Persani L, Conti M (2008) Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol 22(4):924–936. doi:10.1210/me.2007-0246
Diaz F, Wigglesworth K, Eppig J (2007) Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci 120(8):1330–1340
Eppig JJ, Pendola FL, Wigglesworth K (1998) Mouse oocytes suppress cAMP-induced expression of LH receptor messenger RNA by granulosa cells in vitro. Mol Reprod Dev 49:327–332
Y-Q S, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ (2002) Mitogen-activated protein kinase activity in cumulus cells is essential for gonadotropin-induced oocyte meiotic resumption and cumulus expansion in the mouse. Endocrinology 143(6):2221–2232
Y-Q S, Denegre JM, Wigglesworth K, Pendola FL, O'Brien MJ, Eppig JJ (2003) Oocyte-dependent activation of mitogen-activated protein kinase (ERK1/2) in cumulus cells is required for the maturation of the mouse oocyte-cumulus cell complex. Dev Biol 263(1):126–138
Diaz FJ, O'Brien MJ, Wigglesworth K, Eppig JJ (2006) The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol 299(1):91–104
Jamnongjit M, Gill A, Hammes SR (2005) Epidermal growth factor receptor signaling is required for normal ovarian steroidogenesis and oocyte maturation. Proc Natl Acad Sci U S A 102(45):16257–16262
Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA (2008) Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin 43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 135(19):3229–3238. doi:10.1242/dev.025494
Diaz FJ, Anderson LE, YL W, Rabot A, Tsai SJ, Wiltbank MC (2002) Regulation of progesterone and prostaglandin F2alpha production in the CL. Mol Cell Endocrinol 191(1):65–80
Kim AM, Vogt S, O'Halloran TV, Woodruff TK (2010) Zinc availability regulates exit from meiosis in maturing mammalian oocytes. Nat Chem Biol 6(9):674–681. doi:10.1038/nchembio.419
Tian X, Diaz FJ (2012) Zinc depletion causes multiple defects in ovarian function during the periovulatory period in mice. Endocrinology 153(2):873–886
Bernhardt ML, Kim AM, O'Halloran TV, Woodruff TK (2011) Zinc requirement during meiosis I–meiosis II transition in mouse oocytes is independent of the MOS-MAPK pathway. Biol Reprod 84(3):526–536. doi:10.1095/biolreprod.110.086488
Tian X, Anthony K, Neuberger T, Diaz FJ (2014) Preconception zinc deficiency disrupts postimplantation fetal and placental development in mice. Biol Reprod 90(4):83. doi:10.1095/biolreprod.113.113910
Tian X, Diaz FJ (2013) Acute dietary zinc deficiency before conception compromises oocyte epigenetic programming and disrupts embryonic development: preconception zinc and oocyte quality. Dev Biol 376(1):51–61. doi:10.1016/j.ydbio.2013.01.015
Diaz FJ, Luo W, Wiltbank MC (2011) Effect of decreasing intraluteal progesterone on sensitivity of the early porcine corpus luteum to the luteolytic actions of prostaglandin F2alpha. Biol Reprod 84(1):26–33. doi:10.1095/biolreprod.110.084368
Clegg M, Keen C, Lönnerdal B, Hurley L (1981) Influence of ashing techniques on the analysis of trace elements in animal tissue. Biol Trace Elem Res 3(2):107–115. doi:10.1007/BF02990451
Diaz FJ, Wiltbank MC (2004) Acquisition of luteolytic capacity: changes in prostaglandin F 2alpha regulation of steroid hormone receptors and estradiol biosynthesis in pig corpora lutea. Biol Reprod 70(5):1333–1339
Diaz FJ, Crenshaw TD, Wiltbank MC (2000) Prostaglandin f(2alpha) induces distinct physiological responses in porcine corpora lutea after acquisition of luteolytic capacity. Biol Reprod 63(5):1504–1512
Diaz FJ, Wigglesworth K, Eppig JJ (2007) Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev Biol 305(1):300–311
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta]CT method. Methods 25(4):402–408
Graham JD, Clarke CL (1997) Physiological action of progesterone in target tissues. Endocr Rev 18(4):502–519
Conneely OM, Mulac-Jericevic B, Lydon JP (2003) Progesterone-dependent regulation of female reproductive activity by two distinct progesterone receptor isoforms. Steroids 68(10–13):771–778. doi:10.1016/S0039-128X(03)00126-0
Li R, Norman RJ, Armstrong DT, Gilchrist RB (2000) Oocyte-secreted factor(s) determine functional differences between bovine mural granulosa cells and cumulus cells. Biol Reprod 63(3):839–845
Motola S, Popliker M, Tsafriri A (2007) Are steroids obligatory mediators of luteinizing hormone/human chorionic gonadotropin-triggered resumption of meiosis in mammals? Endocrinology 148(9):4458–4465. doi:10.1210/en.2007-0445
Zheng P, Si W, Bavister BD, Yang J, Ding C, Ji W (2003) 17{beta}-Estradiol and progesterone improve in-vitro cytoplasmic maturation of oocytes from unstimulated prepubertal and adult rhesus monkeys. Hum Reprod 18(10):2137–2144. doi:10.1093/humrep/deg410
Flores-Herrera H, Díaz-Cervantes P, De la Mora G, Zaga-Clavellina V, Uribe-Salas F, Castro I (2008) A possible role of progesterone receptor in mouse oocyte in vitro fertilization regulated by norethisterone and its reduced metabolite. Contraception 78(6):507–512
Park OK, Mayo KE (1991) Transient expression of progesterone receptor messenger RNA in ovarian granulosa cells after the preovulatory luteinizing hormone surge. Mol Endocrinol 5(7):967–978
Natraj U, Richards JS (1993) Hormonal regulation, localization, and functional activity of the progesterone receptor in granulosa cells of rat preovulatory follicles. Endocrinology 133(2):761–769
Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS (2000) Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci U S A 97(9):4689–4694
Peluso JJ (2006) Multiplicity of progesterone’s actions and receptors in the mammalian ovary. Biol Reprod 75(1):2–8. doi:10.1095/biolreprod.105.049924
Peluso JJ (2007) Non-genomic actions of progesterone in the normal and neoplastic mammalian ovary. Semin Reprod Med 25(03):198–207
Robker RL, Richards JS (2000) Progesterone: lessons from the progesterone receptor knockout. In: Adashi E, Stouffer R (eds) Ovulation: evolving scientific and clinical concepts. Springer, New York, pp. 121–129
Qiu HB, SS L, Ji KL, Song XM, YQ L, Zhang M, KH L (2008) Membrane progestin receptor beta (mPR-[beta]): a protein related to cumulus expansion that is involved in in vitro maturation of pig cumulus-oocyte complexes. Steroids 73(14):1416–1423
Peluso JJ, Pappalardo A, Losel R, Wehling M (2006) Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone’s antiapoptotic action. Endocrinology 147(6):3133–3140. doi:10.1210/en.2006-0114
Luciano AM, Lodde V, Franciosi F, Ceciliani F, Peluso JJ (2010) Progesterone receptor membrane component 1 expression and putative function in bovine oocyte maturation, fertilization, and early embryonic development. Reproduction 140(5):663–672. doi:10.1530/rep-10-0218
Sugiura K, Su YQ, Diaz FJ, Pangas SA, Sharma S, Wigglesworth K, O’Brien MJ, Matzuk MM, Shimasaki S, Eppig JJ (2007) Oocyte-derived BMP15 and FGFs cooperate to promote glycolysis in companion cumulus cells. Development 134(14):2593–2603
Chang H-M, Cheng J-C, Klausen C, Leung PCK (2013) BMP15 suppresses progesterone production by down-regulating StAR via ALK3 in human granulosa cells. Mol Endocrinol 27(12):2093–2104. doi:10.1210/me.2013-1233
Fang L, Chang H-M, Cheng J-C, Leung PC, Sun Y-P (2014) TGF-β1 downregulates StAR expression and decreases progesterone production through Smad3 and ERK1/2 signaling pathways in human granulosa cells. The Journal of Clinical Endocrinology & Metabolism 99(11):E2234–E2243. doi:10.1210/jc.2014-1930
Miro F, Smyth CD, Hillier SG (1991) Development-related effects of recombinant activin on steroid synthesis in rat granulosa cells. Endocrinology 129(6):3388–3394. doi:10.1210/endo-129-6-3388
Clem BF, Clark BJ (2006) Association of the mSin3A-histone deacetylase 1/2 corepressor complex with the mouse steroidogenic acute regulatory protein gene. Mol Endocrinol 20(1):100–113. doi:10.1210/me.2004-0495
Kuo F-T, Fan K, Bentsi-Barnes I, Barlow GM, Pisarska MD (2012) Mouse forkhead L2 maintains repression of FSH-dependent genes in the granulosa cell. Reproduction 144(4):485–494. doi:10.1530/rep-11-0259
Lisle R, Anthony K, Randall M, Diaz F (2013) Oocyte-cumulus cell interactions regulate free intracellular zinc in mouse oocytes. Reproduction 145:381–390
Arslan P, Di Virgilio F, Beltrame M, Tsien RY, Pozzan T (1985) Cytosolic Ca2+ homeostasis in Ehrlich and Yoshida carcinomas. A new, membrane-permeant chelator of heavy metals reveals that these ascites tumor cell lines have normal cytosolic free Ca2+. J Biol Chem 260(5):2719–2727
Martell A, Smith R (2004) NIST critical stability constants of metal complexes (, 1998). NIST Standard Reference Database 46, v80 Gaithersburg, MD: Standard Reference Data Program, National Institute of Standards and Technology, U.S. Dept. of Commerce
Kolesarova A, Capcarova M, Medvedova M, Sirotkin AV, Kovacik J (2011) In vitro assessment of iron effect on porcine ovarian granulosa cells: secretory activity, markers of proliferation and apoptosis. Physiol Res 60(3):503–510
Strushkevich N, MacKenzie F, Cherkesova T, Grabovec I, Usanov S, Park H-W (2011) Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc Natl Acad Sci 108(25):10139–10143. doi:10.1073/pnas.1019441108
Roychoudhury S, Bulla J, Sirotkin AV, Kolesarova A (2014) In vitro changes in porcine ovarian granulosa cells induced by copper. J Environ Sci Health, Part A: Tox Hazard Subst Environ Eng 49(6):625–633. doi:10.1080/10934529.2014.865404
Acknowledgments
We thank Dr. Doug Stocco for providing STAR antibody and to Nick McCormick, Tom Croxford, and Shannon Kelleher for help with tissue zinc analyses.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ᅟ
Animals were maintained according to the Guide for the Care and Use of Laboratory Animals (Institute for Learning and Animal Research). All animal use was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the Pennsylvania State University.
Conflict of Interest
The authors declare that they have no conflict of interest.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Research Involving Animal Use
All procedures used on animals were approved by the Animal Care and Use Committee of the Pennsylvania State University.
Rights and permissions
About this article
Cite this article
Tian, X., Anthony, K. & Diaz, F.J. Transition Metal Chelator Induces Progesterone Production in Mouse Cumulus-Oocyte Complexes and Corpora Lutea. Biol Trace Elem Res 176, 374–383 (2017). https://doi.org/10.1007/s12011-016-0841-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0841-x