Abstract
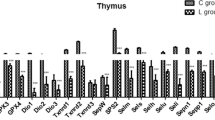
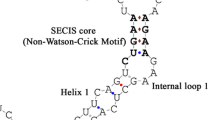
Selenoprotein T (SelT) is associated with the regulation of calcium homeostasis and neuroendocrine secretion. SelT can also change cell adhesion and is involved in redox regulation and cell fixation. However, the structure and function of chicken SelT and its response to selenium (Se) remains unclear. In the present study, 150 1-day-old chickens were randomly divided into a low Se group (L group, fed a Se-deficient diet containing 0.020 mg/kg Se) and a control group (C group, fed a diet containing sodium selenite at 0.2 mg/kg Se). The immune organs (spleen, thymus, and bursa of Fabricius) were collected at 15, 25, 35, 45, and 55 days of age. We performed a sequence analysis and predicted the structure and function of SelT. We also investigated the effects of Se deficiency on the expression of SelT, selenophosphate synthetase-1 (SPS1), and selenocysteine synthase (SecS) using RT-PCR and the oxidative stress in the chicken immune organs. The data showed that the coding sequence (CDS) and deduced amino acid sequence of SelT were highly similar to those of 17 other animals. Se deficiency induced lower (P < 0.05) levels of SelT, SPS1, and SecS, reduced the catalase (CAT) activity, and increased the levels of hydrogen peroxide (H2O2) and hydroxyl radical (–OH) in immune organs. In conclusion, the CDS and deduced amino acid sequence of chicken SelT are highly homologous to those of various mammals. The redox function and response to the Se deficiency of chicken SelT may be conserved. A Se-deficient diet led to a decrease in SelT, SecS, and SPS1 and induced oxidative stress in the chicken immune organs. To our knowledge, this is the first report of predictions of chicken SelT structure and function. The present study demonstrated the relationship between the selenoprotein synthases (SPS1, SecS) and SelT expression in the chicken immune organs and further confirmed oxidative stress caused by Se deficiency. Thus, the information presented in this study is helpful to understand chicken SelT structure and function. Meanwhile, the present research also confirmed the negative effects of Se deficiency on chicken immune organs.











Similar content being viewed by others
References
Lenz M, Lens PN (2009) The essential toxin: the changing perception of selenium in environmental sciences. Sci Total Environ 407(12):3620–3633
Nazıroğlu M, Yıldız K, Tamtürk B et al (2012) Selenium and psoriasis. Biol Trace Elem Res 150(1–3):3–9
Fatmi W, Kechrid Z, Nazıroğlu M et al (2013) Selenium supplementation modulates zinc levels and antioxidant values in blood and tissues of diabetic rats fed zinc-deficient diet. Biol Trace Elem Res 152(2):243–250
Lei C, Niu X, Ma X et al (2011) Is selenium deficiency really the cause of Keshan disease? Environ Geochem Health 33(2):183–188
Robinson M, Campbell D, Sutherland W et al (1983) Selenium and risk factors for cardiovascular disease in New Zealand. New Zealand Med J 96(741):755–757
Demirci S, Kutluhan S, Nazıroğlu M et al (2013) Effects of selenium and topiramate on cytosolic Ca2+ influx and oxidative stress in neuronal PC12 cells. Neurochem Res 38(1):90–97
McKenzie RC, Rafferty TS, Beckett GJ (1998) Selenium: an essential element for immune function. Immunol Today 19(8):342–345
Hoffmann PR, Berry MJ (2008) The influence of selenium on immune responses. Mol Nutr Food Res 52(11):1273–1280
Wilson CL, Mann J, Walsh M et al (2014) Quiescent hepatic stellate cells functionally contribute to the hepatic innate immune response via TLR3. PLoS One 9(1):e83391
Oda SS, El-Maddawy ZK (2012) Protective effect of vitamin E and selenium combination on deltamethrin-induced reproductive toxicity in male rats. Exp Toxicol Pathol 64(7):813–819
Willett W, Steven Morris J, Pressel S et al (1983) Prediagnostic serum selenium and risk of cancer. Lancet 322(8342):130–134
Hoffmann PR (2007) Mechanisms by which selenium influences immune responses. Arch Immunol Ther Exp 55(5):289–297
Papp LV, Lu J, Holmgren A et al (2007) From selenium to selenoproteins: synthesis, identity, and their role in human health. Antioxid Redox Signal 9(7):775–806
Leinfelder W, Zehelein E, MandrandBerthelot M et al (1988) Gene for a novel tRNA species that accepts L-serine and cotranslationally inserts selenocysteine
Hatfield DL, Tsuji PA, Carlson BA et al (2014) Selenium and selenocysteine: roles in cancer, health, and development. Trends in Biochemical Sciences
Kryukov GV, Castellano S, Novoselov SV et al (2003) Characterization of mammalian selenoproteomes. Science 300(5624):1439–1443
Reeves M, Hoffmann P (2009) The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci 66(15):2457–2478
Pappas A, Zoidis E, Surai P et al (2008) Selenoproteins and maternal nutrition. Comp Biochem Physiol B Biochem Mol Biol 151(4):361–372
Grumolato L, Ghzili H, Montero-Hadjadje M et al (2008) Selenoprotein T is a PACAP-regulated gene involved in intracellular Ca2+ mobilization and neuroendocrine secretion. FASEB J 22(6):1756–1768
Dikiy A, Novoselov SV, Fomenko DE et al (2007) SelT, SelW, SelH, and Rdx12: genomics and molecular insights into the functions of selenoproteins of a novel thioredoxin-like family. Biochemistry 46(23):6871–6882
Hoffmann PR, Höge SC, Li P-A et al (2007) The selenoproteome exhibits widely varying, tissue-specific dependence on selenoprotein P for selenium supply. Nucleic Acids Res 35(12):3963–3973
Peirson SN, Butler JN, Foster RG (2003) Experimental validation of novel and conventional approaches to quantitative real-time PCR data analysis. Nucleic Acids Res 31(14):e73–e73
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29(9):e45–e45
Weissman SM (1976) Red cell metabolism. a manual of biochemical methods. Yale J Biol Med 49(3):310
Zhou B, Wang J, Guo Z et al (2006) A simple colorimetric method for determination of hydrogen peroxide in plant tissues. Plant Growth Regul 49(2–3):113–118
Pascual C, Romay C (1992) Effect of antioxidants on chemiluminescence produced by reactive oxygen species. J Biolumin Chemilumin 7(2):123–132
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254
Sengupta A, Carlson BA, Labunskyy VM et al (2009) Selenoprotein T deficiency alters cell adhesion and elevates selenoprotein W expression in murine fibroblast cells. Biochem Cell Biol 87(6):953–961
Aachmann FL, Fomenko DE, Soragni A et al (2007) Solution structure of selenoprotein W and NMR analysis of its interaction with 14-3-3 proteins. J Biol Chem 282(51):37036–37044
Yao H-D, Wu Q, Zhang Z-W et al (2013) Gene expression of endoplasmic reticulum resident selenoproteins correlates with apoptosis in various muscles of SE-deficient chicks. J Nutr 143(5):613–619
Huang J-Q, Li D-L, Zhao H et al (2011) The selenium deficiency disease exudative diathesis in chicks is associated with downregulation of seven common selenoprotein genes in liver and muscle. J Nutr 141(9):1605–1610
Sun B, Wang R, Li J et al (2011) Dietary selenium affects selenoprotein W gene expression in the liver of chicken. Biol Trace Elem Res 143(3):1516–1523
Schmidt RL, Simonović M (2012) Synthesis and decoding of selenocysteine and human health. Croat Med J 53(6):535–550
Wang R, Sun B, Zhang Z et al (2011) Dietary selenium influences pancreatic tissue levels of selenoprotein W in chickens. J Inorg Biochem 105(9):1156–1160
Xu X, Carlson B, Irons R et al (2007) Selenophosphate synthetase 2 is essential for selenoprotein biosynthesis. Biochem J 404:115–120
Wang K-T, Wang J, Li L-F et al (2009) Crystal structures of catalytic intermediates of human selenophosphate synthetase 1. J Mol Biol 390(4):747–759
Özcelik D, Nazıroglu M, Tunçdemir M et al (2012) Zinc supplementation attenuates metallothionein and oxidative stress changes in kidney of streptozotocin-induced diabetic rats. Biol Trace Elem Res 150(1–3):342–349
Massafra C, Gioia D, De Felice C et al (2000) Effects of estrogens and androgens on erythrocyte antioxidant superoxide dismutase, catalase and glutathione peroxidase activities during the menstrual cycle. J Endocrinol 167(3):447–452
Yu D, Zhang Z-w, Yao H-d et al (2014) Antioxidative role of selenoprotein W in oxidant-induced chicken splenic lymphocyte death. BioMetals 27(2):277–291
Han Y-H, Zhang Z-W, Su J et al (2012) Effects of chicken selenoprotein W on H2O2-induced apoptosis in CHO-K1 cells. Biol Trace Elem Res 147(1–3):395–402
Zhang Z-w, Wang Q-h, Zhang J-l et al (2012) Effects of oxidative stress on immunosuppression induced by selenium deficiency in chickens. Biol Trace Elem Res 149(3):352–361
Pigeolet E, Corbisier P, Houbion A et al (1990) Glutathione peroxidase, superoxide dismutase, and catalase inactivation by peroxides and oxygen derived free radicals. Mech Ageing Dev 51(3):283–297
Tate D, Miceli MV, Newsome DA (1995) Phagocytosis and H2O2 induce catalase and metallothionein gene expression in human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 36(7):1271–1279
Hansford RG, Hogue BA, Mildaziene V (1997) Dependence of H2O2 formation by rat heart mitochondria on substrate availability and donor age. J Bioenerg Biomembr 29(1):89–95
Nazıroğlu M (2012) Molecular role of catalase on oxidative stress-induced Ca2+ signaling and TRP cation channel activation in nervous system. J Recept Sig Transd 32(3):134–141
Acknowledgments
This research is supported by the National Natural Science Foundation of China (Grant No. 31320103920).
Conflict of Interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
You, L., Liu, C., Yang, Zj. et al. Prediction of Selenoprotein T Structure and Its Response to Selenium Deficiency in Chicken Immune Organs. Biol Trace Elem Res 160, 222–231 (2014). https://doi.org/10.1007/s12011-014-0049-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0049-x