Abstract
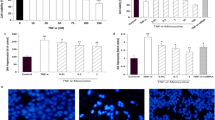
Zinc (Zn), a cell-protective metal against various toxic compounds, is the key agent for neutral endopeptidase (NEP) functional structure. NEP is a zinc metalloenzyme which degrades endogenous opioids and is expressed in human keratinocytes (HaCaT). Ropivacaine, a widely used opiate local anaesthetic, exerts cell toxic and apoptotic effects against HaCaT cells. The aim of the present study is to investigate whether zinc modulates the effects of ropivacaine on proliferation, viability, apoptosis and NEP expression in HaCaT cells. To investigate the role of ropivacaine in NEP function, HaCaT cells overexpressing NEP were generated via cell transfection with plasmids carrying NEP cDNA. Ropivacaine's anti-proliferative effect was tested by Neubauer's chamber cell counting, and induction of cell death was demonstrated by trypan blue exclusion assay. Apoptosis due to ropivacaine was tested via DNA fragmentation and poly-ADP-ribose-polymerase (PARP) cleavage. NEP and PARP expression was performed by western blot analysis. Results showed that zinc (15 μΜ) inhibited proliferation and cell death induction by ropivacaine (0.5, 1 and 2 mM) (p < 0.05) as well as apoptosis induced by the drug (0.5 and 1 mM) in HaCaT cells. Ropivacaine (1.0, 2.0 and 5.0 mM) downregulated NEP expression in the presence of zinc (15 μΜ) while NEP overexpression enhanced ropivacaine's apoptotic effect. In conclusion, the abilities of zinc to inhibit the toxic and apoptotic effects of ropivacaine, to maintain NEP downregulation induced by the drug and, consequently, to enhance its anaesthetic result suggest that zinc may have a significant role in pain management and tissue protection.






Similar content being viewed by others

References
Owen MD, Dean LS (2000) Ropivacaine. Expert Opin Pharmacother 1:325–336
Werdehausen R, Fazeli S, Braun S et al (2009) Apoptosis induction by different local anaesthetics in a neuroblastoma cell line. Br J Anaesth 103:711–718
Grishko V, Xu M, Wilson G et al (2010) Apoptosis and mitochondrial dysfunction in humanchondrocytes following exposure to lidocaine, bupivacaine and ropivacaine. J Bone Joint Surg Am 92:609–618
Kontargiris E, Kolettas E, Vadalouca A et al (2004) Ectopic expression of clusterin/apolipoprotein J or Bcl-2 decrease the sensitivity of HaCaT cells to toxic effects of ropivacaine. Cell Res 14:415–422
Perry DK, Smyth MJ, Stennicke HR et al (1997) Zinc is a potent inhibitor of the apoptotic protease, caspase-3. A novel target for zinc in the inhibition of apoptosis. J Biol Chem 272:18530–18533
Zalewski PD, Forbes IJ, Giannakis C (1991) Physiological role for zinc in prevention of apoptosis (gene-directed death). Biochem Int 24:1093–1101
Tamura T, Sadakata N, Oda T et al (2002) Role of zinc ions in ricin-induced apoptosis in U937 cells. Toxicol Lett 132:141–151
Ganju N, Eastman A (2003) Zinc inhibits Bax and Bak activation and cytochrome c release induced by chemical inducers of apoptosis but not by death-receptor-initiated pathways. Cell Death Differ 10:652–661
Wei Q, Wang J, Wang MH et al (2004) Inhibition of apoptosis by Zn2+ in renal tubular cells following ATP-depletion. Am J Physiol Renal Physiol 287:F492–F500
Tang H-B, Miyano K, Nakata Y (2009) Modulation of the substance P release from cultured rat primary afferent neurons by zinc ions. J Pharmacol Sci 110:397–400
Qian J, Noebels JL (2005) Visualization of transmitter release with zinc fluorescence detection at the mouse hippocampal mossy fibre synapse. J Physiol 566:747–758
Roques BP, Noble F, Daugè V et al (1993) Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol Rev 45:87–146
Noble F, Roques BP (2007) Protection of endogenous enkephalin catabolism as natural approach to novel analgesic and antidepressant drugs. Expert Opin Ther Targets 11:145–159
Erdös EG, Skidgel RA (1989) Neutral endopeptidase 24.11 (enkephalinase) and related regulators of peptide hormones. FASEB 3:145–151
Wisner A, Dufour E, Messaoudi M et al (2006) Human opiorphin, a natural antinociceptive modulator of opioid-dependent pathways. Proc Natl Acad Sci U S A 103:17979–17984
Thanawala V, Kadam VJ, Ghosh R (2008) Enkephalinase inhibitors: potential agents for the management of pain. Curr Drug Targets 9:887–894
Papandreou CN, Usmani B, Geng Y et al (1998) Neutral endopeptidase 24.11 loss in metastatic human prostate cancer contributes to androgen independent progression. Nat Med 4:50–57
Sumitomo M, Asano T, Asakuma J (2004) Chemosensitization of androgen-independent prostate cancer with neutral endopeptidase. Clin Cancer Res 10:260–266
Kajiyama H, Shibata K, Terauchi M (2005) Neutral endopeptidase 24.11/DC 10 suppresses progressive potential in ovarian carcinoma in vitro and in vivo. Clin Cancer Res 11:1798–1808
Boukamp P, Petrussevska RT, Breitkreutz D (1988) Normal keratinisation in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 106:761–771
Lanctot C, Fournier H, Howell S et al (1995) Direct targeting of neutral endopeptidase (EC 3.4.24.11) to the apical cell surface of transfected LLC-PK1 cells and unpolarized secretion of its soluble form. Biochem J 305:165–171
Gonos ES, Spandidos DA (1993) Oncogenes in cellular immortalization and differentiation. Anticancer Res 13:1117–1122
Vianale G, Reale M, Amerio P et al (2008) Extremely low frequency electromagnetic field enhances human keratinocyte cell growth and decreases proinflammatory chemokine production. Br J Dermatol 158:1189–1196
Turesson I, Nyman J, Qvarnström F et al (2010) A low-dose hypersensitive keratinocyte loss in response to fractionated radiotherapy is associated with growth arrest and apoptosis. Radiother Oncol 94:90–101
Gorman L, Mercer LP, Henning B (1996) Growth requirements of endothelial cells in culture: variation in serum and amino acids concentrations. Nutrition 12:266–270
Sugiki H, Hozumi Y, Maeshima H (2000) C2-ceramide induces apoptosis in a human squamous cell carcinoma cell line. Br J Dermatol 143:1154–1163
Brower MC, Johnson ME (2003) Adverse effects of local anesthetic infiltration on wound healing. Reg Anesth Pain Med 28:233–240
Lansdown AB, Mirastschijski U, Stubbs N et al (2007) Zinc in wound healing: theoretical experimental and clinical aspects. Wound Repair Regen 15:2–16
Sharir H, Zinger A, Nevo A et al (2010) Zinc released from injured cells is acting via the Zn2+-sensing receptor, ZnR, to trigger signaling leading to epithelial repair. J Biol Chem 285:26097–26106
Sekler I, Sensi SL, Hershfinkel M et al (2007) Mechanism and regulation of cellular zinc transport. Mol Med 13:337–343
Kalfakakou VP, Evangelou AM, Benveniste J et al (1993) The effects of Zn2+ on guinea pig isolated heart preparations. Biol Trace Elem Res 38:289–299
Evangelou A, Kalfakakou V, Benveniste J et al (1995) Inhibition of PAF-acether effects on isolated guinea pig hearts by zinc ions. Biol Trace Elem Res 50:43–55
Rougeot C, Messaoudi M, Hermitte V et al (2003) Sialorphin, a natural inhibitor of rat membrane-bound neutral endopeptidase that displays analgesic activity. Proc Natl Acad Sci U S A 100:8549–8554
Nissen JB, Kragballe K (1997) Enkephalins modulate differentiation of normal human keratinocytes in vitro. Exp Dermatol 6:222–229
Cioca DP, Kitano K (2002) Induction of apoptosis and CD10/neutral endopeptidase expression by jaspamide in HL-60 line cells. Cell Mol Life Sci 59:1377–1387
Dai J, Shen R, Sumitomo M (2001) Tumor-suppressive effects of neutral endopeptidase in androgen-independent prostate cancer cells. Clin Cancer Res 7:1370–1377
Acknowledgments
This work was partially supported by a European's Society for Regional Anaesthesia (ESRA) Research Grant.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kontargiris, E., Vadalouka, A., Ragos, V. et al. Zinc Inhibits Apoptosis and Maintains NEP Downregulation, Induced by Ropivacaine, in HaCaT Cells. Biol Trace Elem Res 150, 460–466 (2012). https://doi.org/10.1007/s12011-012-9492-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9492-8