Abstract
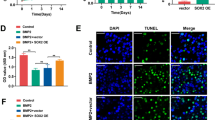
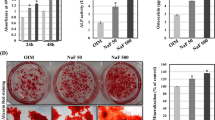
A series of experimental methods including 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide, alkaline phosphatase (ALP) activity measurement, alizarin red S stain and measurement, quantitative real-time reverse transcriptase polymerase chain reaction, and Western blot analysis were employed to assess the effects of LaCl3 on the proliferation, osteogenic differentiation, and mineralization of a murine preosteoblast cell line MC3T3-E1 at cell and molecular levels. The results indicated that LaCl3 had dual effects on the proliferation, osteogenic differentiation, and mineralization of MC3T3-E1 cells. First, LaCl3 promoted the proliferation, osteogenic differentiation, and mineralization of MC3T3-E1 cells at lower concentrations, then had no effects and further turned to inhibit the proliferation, osteogenic differentiation, and mineralization of MC3T3-E1 cells with increasing concentrations. The expression of runt-related transcription factor 2 (Runx2), bone morphogenetic protein 2 (BMP2), ALP, bone sialoprotein (BSP), collagen I (Col I), and osteocalcin (OCN) genes was upregulated in the presence of 0.0001 and 0.1 μM LaCl3, but these genes were downregulated in the MC3T3-E1 cells treated with 1,000 μM LaCl3. In addition, the expression of BMP2, Runx2, and OCN proteins was promoted by LaCl3 at the concentration of 0.0001 μM, but these proteins were downregulated after 1,000 μM LaCl3 treatment. The results suggest that LaCl3 likely up- or downregulates the expression of Runx2, which subsequently up- or downregulates osteoblasts marker genes Col I and BMP2 at early stages and ALP and OCN at later stages of differentiation, thus causes to promote or inhibit the proliferation, osteogenic differentiation and mineralization of MC3T3-E1 cells. The results will be helpful for understanding the mechanisms of bone metabolism and application of lanthanum-based compounds in the future.





Similar content being viewed by others
Abbreviations
- ARS:
-
Alizarin red S
- ALP:
-
Alkaline phosphatase
- α-MEM:
-
Alpha minimum essential medium
- BMP2:
-
Bone morphogenetic protein 2
- BSP:
-
Bone sialoprotein
- Col I:
-
Collagen I
- cDNA:
-
Complementary DNA
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
- ECL:
-
Enhanced chemiluminescene
- EDTA:
-
Ethylenediaminetetraacetic acid tetrasodium salt
- FBS:
-
Fetal bovine serum
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- La:
-
Lanthanum
- OD:
-
Optical density
- OBs:
-
Osteoblasts
- OCN:
-
Osteocalcin
- OS:
-
Osteogenetic induction supplement
- Q-PCR:
-
Quantitative real-time reverse transcriptase polymerase chain reaction
- Runx2:
-
Runt-related transcription factor 2
References
Kramsch DM, Aspen AJ, Apstein CS (1980) Suppression of experimental atherosclerosis by the Ca2+-antagonist lanthanum. Possible role of calcium in atherogenesis. J Clin Invest 65(5):967–981
Fricker SP (2006) The therapeutic application of lanthanides. Chem Soc Rev 35:524–533
Li RC, Yang HW, Wang K (2003) La accumulation and microstructure change of leg bones of rats fed with La(NO3)3 in low dosage for a long term. J Peking Univ (Health Sci) 35(6):622–624
Huang J, Zhang TL, Xu SJ (2006) Effects of lanthanum on composition, crystal size and lattice structure of femur bone mineral of Wistar rats. Calcif Tissue Int 78(4):241–247
Zhang JC, Xu SJ, Wang K et al (2003) Effects of the rare earth ions on bone resorbing function of rabbit mature osteoclasts in vitro. Chin Sci Bull 48(20):2170–2175
Wang X, Yuan L, Huang J et al (2008) Lanthanum enhances in vitro osteoblast differentiation via pertussis toxin-sensitive gi protein and ERK signaling pathway. J Cell Biochem 105(5):1307–1315
Shi YL, Wang LW, Huang J et al (2009) Lanthanum suppresses osteoblastic differentiation via pertussis toxin-sensitive G protein signaling in rat vascular smooth muscle cells. J Cell Biochem 108(5):1184–1191
Zhang DW, Zhang JC, Chen Y et al (2007) Effects of lanthanum and gadolinium on proliferation and differentiation of primary osteoblasts. Prog Nat Sci 17(5):618–623
Wang D, Christensen K, Chawla K et al (1999) Isolation and characterization of MC3T3-E1 preosteoblast subclones with distinct in vitro and in vivo differentiation/mineralization potential. Bone Miner Res 14(6):893–903
Carmichael J, Degraff WG, Gazdar AF et al (1987) Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res 47(4):936–942
Zhao Y, Zou B, Shi ZY et al (2007) The effect of 3-hydroxybutyrate on the in vitro differentiation of murine osteoblast MC3T3-E1 and in vivo bone formation in ovariectomized rats. Biomaterials 28(20):3063–3073
Gori F, Divieti P, Demay M (2001) Cloning and characterization of a novel WD-40 repeat protein that dramatically accelerates osteoblastic differentiation. J Biol Chem 276(49):46515–46522
Liu DD, Yi CQ, Zhang DW et al (2010) Inhibition of proliferation and differentiation of mesenchymal stem cells by carboxylated carbon nanotubes. ACS Nano 4:2185–2195
Zhang JC, Li XX, Xu SJ et al (2004) Effects of rare earth ions on proliferation, differentiation and function expression of cultured osteoblasts in vitro. Prog Nat Sci 14(4):404–409
Zhang JC, Liu CL, Li YP et al (2010) Effect of cerium ion on the proliferation, differentiation and mineralization function of primary mouse osteoblasts in vitro. J Rare Earths 28(1):138–142
Zhang JC, Liu CL, Li YP et al (2010) Effect of yttrium ion on the proliferation, differentiation and mineralization function of primary mouse osteoblasts in vitro. J Rare Earths 28(3):466–470
Wang K (1997) The analogy in chemical and biological behavior between non-essential ions compared with essential ions. South Afr J Chem 50(4):232–239
Mu QX, Du GQ, Chen TS et al (2009) Suppression of human bone mophorgenetic protein (BMP) signaling by carboxylated single-walled carbon nanotubes. ACS Nano 3:1139–1144
Ducy P, Zhang R, Geoffroy V et al (1997) Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell 89(5):747–754
Nakashima K, Zhou X, Kunkel G et al (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108(1):17–29
Beertsen W, Van Den Bos T (1991) Alkaline phosphatase induces the deposition of calcified layers in relation to dentin: an in vitro study to mimic the formation of afibrillar acellular cementum. J Dent Res 70(3):176–181
Chou YF, Dunn JCY, Wu BM (2005) In vitro response of MC3T3-E1 preosteoblasts within three-dimensional apatite-coated PLGA scaffolds. J Biomedic Mater Res 75B(1):81–90
Komori T, Yagi H, Nomura S et al (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89(5):755–764
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 20971034), Natural Science Key Foundation of Hebei Province (no. B2009000161), Research Fund for the Doctoral Program of Higher Education of China (no.20111301110004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, D., Zhang, J., Wang, G. et al. The Dual-Effects of LaCl3 on the Proliferation, Osteogenic Differentiation, and Mineralization of MC3T3-E1 Cells. Biol Trace Elem Res 150, 433–440 (2012). https://doi.org/10.1007/s12011-012-9486-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9486-6