Abstract
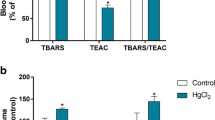
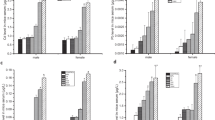
Erythrocytes are a convenient model to understand the subsequent oxidative deterioration of biological macromolecules in metal toxicities. The present study examined the variation of hematoxic and genotoxic parameters following subchronic exposure of mercuric chloride via drinking water and their possible association with oxidative stress. Male rats were exposed to 50 ppm (HG1) and 100 ppm (HG2) of mercuric chloride daily for 90 days. A significant dose-dependent decrease was observed in red blood cell count, hemoglobin, hematocrit, and mean cell hemoglobin concentration in treated groups (HG1 and HG2) compared with controls. A significant dose-dependent increase was observed in lipid peroxidation; therefore, a significant variation was found in the antioxidant enzyme activities, such as superoxide dismutase, catalase, and glutathione peroxidase. Interestingly, mercuric chloride treatment showed a significant dose-dependent increase in frequency of total chromosomal aberration and in percentage of aberrant bone marrow metaphase of treated groups (p < 0.01). The oxidative stress induced by mercury treatment may be the major cause for chromosomal aberration as free radicals lead to DNA damage. These data will be useful in screening the antioxidant activities of natural products, which may be specific to the bone marrow tissue.



Similar content being viewed by others
References
Betti C, Davini T, Barale R (1992) Genotoxic activity of methyl mercury chloride and dimethyl mercury in human lymphocytes. Mutat Res 281:255–260
Woods JS, Calas CA, Aicher LD, Robinson BH, Maler C (1990) Stimulation of phorfirinogen oxidation by mercuric ion 1. Evidence of free radical formation in the presence of thiols and hydrogen peroxide. Mol Pharmacol 38:253–260
Patni R, Sharma MK, Kumar M (2001) Modulation of certain hematological parameters in mercuric toxicity by Spirulina fusiformis in Swiss albino mice. Indian J Environ Toxicol 11:32–34
Su L, Wang M, Yin ST, Wang HL, Chen L, Sun LG, Ruan DY (2008) The interaction of selenium and mercury in the accumulations and oxidative stress of rat tissues. Ecotoxicol Environ Saf 70:438–489
Park EJ, Park K (2007) Induction of reactive oxygen species and apoptosis in BEAS-2B cells by mercuric chloride. Toxicol in Vitro 21:789–794
Emrah C, Metin A, Ihsan H (2008) Antioxidant effects of methionine, α-lipoic acid, N-acetylcysteine and homocysteine on lead-induced oxidative stress to erythrocytes in rats. Exp Toxicol Pathol 60:289–294
Cimen MY (2008) Free radical metabolism in human erythrocytes. Clin Chim Acta 390:1–11
Oztekin E, Baltaci AK, Tiftik AM, Mogulkoc R (2007) Lipid peroxidation in ovariectomized and pinealectomized rats: the effects of estradiol and progesterone supplementation. Cell Biochem Funct 5:551–554
Mogulkoc R, Baltaci AK, Oztekin E, Aydin L, Sivrikaya A (2006) Melatonin prevents oxidant damage in various tissues of rats with hyperthyroidism. Life Sci 3:311–315
Bediz CS, Baltaci AK, Mogulkoc R, Oztekin E (2006) Zinc supplementation ameliorates electromagnetic field-induced lipid peroxidation in the rat brain. Tohoku J Exp Med 2:133–140
Jadhav SH, Sarkar SN, Tripathi HC (2006) Cytogenetic effects of mixture of selected metals following subchronic exposure through drinking water in male rats. Indian J Exp Biol 44:997–1005
Schurz F, Sabater-Vilar M, Fink-Gremmels J (2000) Mutagenicity of mercury chloride and mechanisms of cellular defence: the role of metal-binding proteins. Mutagenesis 15:525–530
Al-Sabti K, Lloyd DC, Edwards AA, Stenar P (1992) Survey of lymphocyte chromosomal damage in Slovenian workers exposed to occupational clastogens. Mutat Res 280:215–223
Loftenius A, Ekstrand J, Moller E (1997) In vitro effects of mercuric chloride (HgCl2) on human mononuclear cells. Clin Exp Immunol 110:418–422
Topashka-Ancheva M, Metcheva R, Teodorova S (2003) A comparative analysis of the heavy metal loading of small mammals in different regions of Bulgaria II: chromosomal aberrations and blood pathology. Ecotoxicol Environ Saf 54:188–193
Falnoga I, Kregar I, Skreblin M, Tusek-Znidaric M, Stegnar P (1993) Interactions of mercury in rat brain. Biol Trace Elem Res 37:71–83
Oliveira FRT, Ferreira JR, Dos Santos CMC, Macedo LEM, De Olivera RB, Rodrigues JA, Do Nascimento JLM, Faro LRF, Diniz DLWP (2006) Estradiol reduces cumulative mercury and associated disturbances in the hypothalamus–pituitary axis of ovariectomized rats. Ecotoxicol Environ Saf 63:488–493
Boujbiha MA, Hamden K, Guermazi F, Bouslama A, Omezzine A, Kammoun A, El Feki A (2009) Testicular toxicity in mercuric chloride-treated rats: association with oxidative stress. Reprod Toxicol 28:81–89
Shinyashiki M, Kumagai Y, Nakajima H, Nagafune J, Homma-Takeda S, Sagai M, Shimojo N (1998) Differential changes in rat brain nitric oxide synthase in vivo and in vitro by methylmercury. Brain Res 798:147–155
Asada K, Takahashi M, Nagate M (1974) Assay and inhibitors of spinach superoxide dismutase. Agric Biol Chem 38:471–473
Aebi H (1974) Catalase. Meth Enzym Anal 2:673–684
Flohe L, Gunzler WA (1984) Analysis of glutathione peroxidase. Meth Enzymol 105:114–121
Esterbauer H, Gebicki J, Puhl H, Jurgens G (1992) The role of lipid peroxidation and antioxidants in oxidative modification of LDL. Free Radic Biol Med 13:341–390
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Evans EP, Breckon G, Forf CE (1963) An air-drying method for meiotic preparations from mammalian testes. Cytogenetics 3:289–294
Sharma MK, Patni R, Kumar M, Kumar A (2005) Modification of mercury-induced biochemical alterations in blood of Swiss albino mice by Spirulina fusiformis. Environ Toxicol Pharmacol 20:289–296
Valko M, Morris H, Cronin MTD (2005) Metals, toxicity and oxidative stress. Curr Med Chem 12:1161–1208
Gonzalez-Munoz MJ, Meseguer I, Sanchez-Reus MI, Schultz A, Olivero R, Benedí J, Sanchez-Muniz FJ (2008) Beer consumption reduces cerebral oxidation caused by aluminum toxicity by normalizing gene expression of tumor necrotic factor alpha and several antioxidant enzymes. Food Chem Toxicol 3:1111–1118
Tapiero H, Tew KD, Gate L, Machover D (2001) Prevention of pathologies associated with oxidative stress and dietary intake deficiencies: folate deficiency and requirements. Biomed Pharmacother 55:381–390
Cheng YT (1998) Changes of redox status in folic acid deprivation-induced apoptosis: a possible role for homocysteine. MS—thesis, Department of Medical Technology, Graduate Institute of Basic Medicine, Chang Gung University, Taiwan
Harvey AN, Costa ND, Savage JR, Thacker J (1997) Chromosomal aberrations induced by defined DNA double-strand breaks: the origin of achromatic lesions. Somat Cell Mol Genet 23:211–219
Morgan WF, Corcoranj J, Hartmann A, Kaplan MI, Limoli CL, Ponnaiya B (1998) DNA double-strand breaks, chromosomal rearrangements, and genomic instability. Mutat Res 404:125–128
Akiyama M, Oshima H, Nakamura M (2001) Genotoxicity of mercury used in chromosomal aberration tests. Toxicol in Vitro 15:463–467
Rozgaj R, Kasuba V, Blanusa M (2005) Mercury chloride genotoxicity in rats following oral exposure, evaluated by comet assay and micronucleus test. Arch Hiq Rada Toksikol 56:9–15
Ehrenstein C, Shu P, Wickenheiser EB, Hirner AV, Dolfen M, Emons H, Obe G (2002) Methyl mercury uptake and associations with the induction of chromosomal aberrations in Chinese hamster (CHO) cells. Chem Biol Interact 141:259–274
Acknowledgments
This work was supported by the Ministry of Higher Education and Scientific Research in Tunisia.
Conflicts of interest
We declare that we do not have any actual or potential conflict of interest including any financial, personal, or other relationships with other people or organizations concerning this work which will be published in your journal.
Author information
Authors and Affiliations
Corresponding author
Additional information
Mohamed Ali Boujbiha and Ghada Ben Salah participated equally to this work.
Rights and permissions
About this article
Cite this article
Boujbiha, M.A., Ben Salah, G., Ben Feleh, A. et al. Hematotoxicity and Genotoxicity of Mercuric Chloride Following Subchronic Exposure Through Drinking Water in Male Rats. Biol Trace Elem Res 148, 76–82 (2012). https://doi.org/10.1007/s12011-012-9342-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-012-9342-8