Abstract
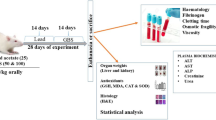
Garlic (Allium sativum) is known to reduce lead toxicity in some species of animals. The objective of this study was to evaluate the efficacy of allicin, one of the most active components of garlic, in the treatment of subacute lead intoxication in sheep. Nine female sheep weighing 25–29 kg orally received a daily dose of 80 mg/kg body weight of lead acetate for 5 days. The animals were then assigned into two groups. Group 1 did not receive any further treatment and was used as the control group and group 2 was treated orally by 2.7 mg/kg body weight of allicin twice daily for 7 days. Within one day following allicin treatment, group 2 blood lead levels were significantly lower than that in group 1 (mean of 616.9 µg/l and 290.02 µg/l, respectively; P < 0.05). Also, allicin treatment significantly reduced kidney lead content and considerably reduced bone and ovary lead contents. These results suggest that allicin might have some therapeutic effects on lead poisoning.
Similar content being viewed by others
References
Radostits OM, Gay CC, Hinchcliff KW, Constable PD (2007) Veterinary medicine, 10th edn. Saunders, London, pp 1799–1808
Chiado LM, Jacobson SW, Jacobson JL (2004) Neurodevelopment effects of postnatal lead exposure at very low levels. Neurotoxicol Teratol 26:359–371
Koller K, Brown T, Spurgeon A, Levy L (2004) Recent development in low-level lead exposure and intellectual impairment in children. Environ Health Perspec 112:987–994
Hoet P, Buchet JP, Decerf L, Lavalleye B, Haufroid V, Lison D (2006) Clinical evaluation of a lead mobilization test using the chelating agent dimercaptosuccinic acid. Clin Chem 52:88–96
Campbell JR, Rosier RN, Novotny L, Puzas JE (2004) The association between environmental lead exposure and bone density in children. Environ Health Perspec 112:1200–1203
Anderson O (1999) Principles and recent developments in chelation treatment of metal intoxication. Chem Rev 99:2683–2710
Osweiler GD (1999) Toxicology, 1st edn. Williams & Wilkins, Baltimore, pp 195–196
Kalia K, Flora SJS (2005) Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. J Occup Health 47:1–21
Meldrum JB, Kok W (2003) Effects of calcium disodium EDTA and meso-2, 3-dimercaptosuccinic acid of tissue concentrations of lead for use in treatment of calves with experimentally induced lead toxicosis. Am J Vet Res 6:672–676
Bratton GR, Zmudzki J, Bell MC, Warnoch LG (1981) Thiamine (vitamin B1) effects on lead intoxication and deposition of lead in tissues: therapeutic potential. Toxicol Appl Pharmacol 59:164–172
Kostial K, Blanusa M, Piasek M, Restek-Samarzija N, Jones MM, Singh PK (1999) Combined chelation therapy in reducing tissue lead concentrations in suckling rats. J Appl Toxicol 19:143–147
Senapati SK, Dey S, Dwivedi SK, Swarup D (2001) Effect of garlic (Allium sativum) extract on tissue lead level in rats. J Ethnopharmacol 76:229–232
Badiei K, Pourjaafar M, Nowrooziasl A (2005) Effect of dried garlic powder (Allium sativum) on lead content of different tissues following subclinical lead poisoning in goats. Iranian J Vet Res 6:12–16
Lawson LD (1996) The composition and chemistry of garlic cloves and processed garlic. In: Koch HP, Lawson LD (eds) Garlic: the science and therapeutic application of Allium sativum L. and related species. 2nd edn. Williams & Wilkins, Baltimore, pp 38–39
Miron T, Bercovici T, Rabinkov A, Wilchek M, Mrelman D (2004) Allicin: preparation and applications. Anal Biochem 331:364–369
Chung LY (2006) The antioxidant properties of garlic compounds: allyl cysteine, alliin, allicin and allyl disulfide. J Med Food 9:205–213
Coppi A, Cabinian M, Mirelman D, Sinnis P (2006) Antimalarial activity of allicin, a biologically active compound from garlic cloves. Antimicrob Agents Chemother 50:1731–1737
Hanafy MS, Shalaby SM, El-Fouly MA (1994) Effect of garlic on lead content in chicken tissues. DTW Dtsch Tieraztl Wonchenschr 10:157–158
Massadeh AM, Al-Safi SA, Momani IF, Alomary AA, Jaradat QM, Alkofahi AS (2007) Garlic (Allium sativum L.) as a potential antidote for cadmium and lead intoxication: cadmium and lead distribution and analysis in different mice organs. Biol Trace Elem Res 120:227–234
Patrick L (2006) Lead toxicity. Part II: the role of free radical damage and the use of antioxidants in the pathology and treatment of lead toxicity. Alt Med Rev 11:114–127
Amagase H, Petesch BL, Matsuura H, Kasuga S, Itakura Y (2001) Intake of garlic and its bioactive compounds. J Nutr 131:955s–962s
Germain E, Auger J, Gines C, Siess MH, Teyssier C (2002) In vivo metabolism of diallyl disulphide in the rat: identification of two new metabolites. Xenobiotica 32:1127–1138
Casteel SW (2006) Lead. In: Peterson ME, Talcott PA (eds) Small Animal Toxicology, 2nd edn. Saunders, St. Louis, pp 795–805
Patrick L (2007) Lead toxicity. A review of the literature. Part I: Exposure, evaluation, and treatment. Alt Med Rev 11:2–22
Kim JS, Blakley BR, Rousseaux CG (1990) The effects of thiamine on tissue distribution of lead. J Appl Toxicol 10:93–97
Kim JS, Hamilton DL, Blakley BR, Roussraux CG (1992) The effect of thiamin on lead metabolism: organ distribution of lead 203. Can J Vet Res 56:256–259
Olkowski AA, Gooneratne SR, Christensen DA (1991) The effects of thiamine and EDTA on biliary and urinary lead excretion in sheep. Toxicol Letter 59:153–159
Maiti SK, Swarup D, Chandra SV (1990) Therapeutic potential of thiamine hydrochloride in experimental chronic lead intoxication in goats. Res Vet Sci 48:377–378
Swarup D, Upadhyay AK (1991) Chemoprophylactic efficacy of thiamine hydrochloride in experimental lead toxicosis in calves. Indian J Anim Sci 61:1170–1173
Coppock RW, Wagner WC, Reynolds JD, Vogel RS, Gelberg HB, Florence LZ, Wolff WA (1991) Evaluation of edentate and thiamine for treatment of experimentally induced environmental lead poisoning in cattle. Am J Vet Res 52:1860–1865
Gwaltney-Beant S (2003) Lead. In: Plumlee KH (ed) Clinical Veterinary Toxicology. Mosby, St. Louis, pp 204–210
Prasad K, Laxdal VA, Yu M, Raney BL (1995) Antioxidant activity of allicin, an active principle in garlic. Molecular cell Biochem 148:183–189
Mousa HM, Al-Qarawi AA, Ali BH, Abdel Rahman HA, Elmougy SA (2002) Effect of lead exposure on the erythrocytic antioxidant levels in goats. J Vet Med A Physiol Clin Med 49:531–534
Anderson BO, Adenuga GA (2007) Effect of lead exposure on liver lipid peroxidative and antioxidant defense systems of protein-undernourished rats. Biol Trace Elem Res 116:219–225
Aykin-Burns N, Laegeler N, Kellogg G, Ercal N (2003) Oxidative effect of lead in young and adult Fisher 344 rats. Arch Environ Contam Toxicol 44:417–420
Taubeneck MW, Domingo JL, Liobet JM, Keen CL (1992) Meso-2,3-dimercaptosuccinic acid (DMSA) affects maternal and fetal copper metabolism in Swiss mice. Toxicol 72:27–40
Domingo JL (1998) Developmental toxicity of metal chelating agents. Reprod Toxicol 12:499–510
Christopher K, Margaret L (2004) Altered mentation caused by polioencephalomalacia, hypernatremia, and lead poisoning. Vet Clin North Am Food Anim Pract 20:287–302
Acknowledgments
The authors wish to thank Dr P. Josling, Nopex-UK, for supplying the allicin, Mr Mohsen Namei Ghasemi, Medical Toxicology Research Center Laboratory, for his assistance in toxicological analysis of the samples and Dr H. Dehghani, Department of Basic Science of our school of Veterinary Medicine, for revision of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Najar-Nezhad, V., Aslani, M.R. & Balali-Mood, M. Evaluation of Allicin for the Treatment of Experimentally Induced Subacute Lead Poisoning in Sheep. Biol Trace Elem Res 126, 141–147 (2008). https://doi.org/10.1007/s12011-008-8185-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-008-8185-9