Abstract
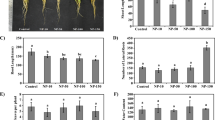
Characterized by a photocatalysis property, nanoanatase is closely related to the photosynthesis of spinach. It could not only improve light absorbance, transformation from light energy to electron energy, and active chemical energy, but also promote carbon dioxide (CO2) assimilation of spinach. However, the molecular mechanism of carbon reaction promoted by nanoanatase remains largely unclear. In this study, we report that the amounts of Rubisco activase (rca) mRNA in the nanoanatase-treated spinach were increased by about 51%, whereas bulk-TiO2 treatment produced an increase of only 5%. Accordingly, the protein level of Rubisco activase from the nanoanatase-treated spinach was increased by 42% compared with the control; however, bulk-TiO2 treatment resulted in a 5% improvement. Further analysis indicated that the activity of Rubisco activase in the nanoanatase-treated spinach was significantly higher than the control by up to 2.75 times, and bulk-TiO2 treatment had no such significant effects. Together, one of the molecular mechanisms of carbon reaction promoted by nanoanatase is that the nanoanatase treatment results in the enhancement of rca mRNA expressions, protein levels, and activities of Rubisco activase, thereby leading to the improvement of Rubisco carboxylation and the high rate of photosynthetic carbon reaction.





Similar content being viewed by others
References
Crabtree RH (1998) A new type of hydrogen bond. Science 282:2000–2001
Zheng L, Hong FS, Lv SP, Liu C (2005) Effect of nano-TiO2 on strength of naturally aged seeds and growth of spinach. Biol Trace Elem Res 104(1):82–93
Hong FS, Yang P, Gao FQ, Liu C, Zheng L, Yang F, Zhou J (2005) Effect of nano-TiO2 on spectral characterization of photosystem II particles from spinach. Chem Res Chin Univ 21(2):196–200
Hong FS, Zhou J, Liu C, Yang F, Wu C, Zheng L, Yang P (2005) Effect of nano-TiO2 on photochemical reaction of chloroplasts of spinach. Biol Trace Elem Res 105(3):269–280
Hong FS, Yang F, Ma ZN, Zhou J, Liu C, Wu C, Yang P (2005) Influences of nano-TiO2 on the chloroplast ageing of spinach under light. Biol Trace Elem Res 104(3):249–260
Yang F, Hong FS, You WJ, Liu C, Gao FQ, Wu C, Yang P (2006) Influences of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol Trace Elem Res 110(2):179–190
Gao FQ, Hong FS, Liu C, Zheng L, Su MY, Wu X, Yang F, Wu C, Yang P (2006) Mechanism of nano-anatase TiO2 on promoting photosynthetic carbon reaction of spinach: inducing complex of Rubisco–Rubisco activase. Biol Trace Elem Res 111(1–3):239–253
Zheng L, Su MY, Liu C, Chen L, Huang H, Wu X, Liu XQ, Yang F, Gao FQ, Hong FS (2007) Effects of nano-anatase TiO2 on photosynthesis of spinach chloroplasts under different light illumination. Biol Trace Elem Res 119:68–76 DOI 10.1007/s12011-007-0047-3
Su MY, Wu X, Liu C, Qu CX, Liu XQ, Cheng L, Huang H, Hong FS (2007) Promotion of energy transfer and oxygen evolution in spinach photosystem II by nano-anatase TiO2. Biol Trace Elem Res 119:183–192. DOI 10.1007/s12011-007-0065-1
Yang F, Liu C, Gao FQ, Su MY, Wu X, Zheng L, Hong FS, Yang P (2007) The improvement of spinach growth by nano-anatase TiO2 treatment is related to nitrogen photoreduction. Biol Trace Elem Res 119:77–88. DOI 10.1007/s12011-007-0046-4
Su MY, Liu C, Wu X, Liu XQ, Cheng L, Gao FQ, Yang F, Li ZR, Hong FS (2007) Effects of nano-anatase TiO2 on absorption, distribution of light and photochemical activities of chloroplast of spinach. Biol Trace Elem Res 118(2):120–130. DOI 10.1007/s12011-007-0006-2
Gao FQ, Liu C, Qu CX, Zheng L, Yang F, Su MY, Hong FS (2007) Was improvement of spinach growth by nano-TiO2 treatment related to the changes of Rubisco activase? BioMetals (in press). DOI 10.1007/s10534-007-9110-y
Zheng L, Su MY, Wu X, Liu C, Qu CX, Chen L, Huang H, Liu XQ, Hong FS (2007) Effects of nano-anatase on spectral characteristics and distribution of LHC II on the thylakoid membranes of spinach. Biol Trace Elem Res 120(1–3):273–283. DOI 10.1007/s12011-007-8025-3
Zheng L, Su MY, Wu X, Liu C, Qu CX, Chen L, Huang H, Liu XQ, Hong FS (2007) Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol Trace Elem Res DOI 10.1007/s12011-007-8028-0
Hong FS, Liu C, Zheng L, Wang XF, Wu K, Song WP, Lv SP, Tao Y, Zhao GW (2005) Formation of Complexes of Rubisco–Rubisco activase from La3+, Ce3+ treatment spinach. . Sci China Ser B Chem 48(1):67–74
Robert JS (1999) Questions about the complexity of chloroplast ribulose-1,5-bisphosphate carboxylase/oxygenase. Photosynth Res 60:29–42
Keegstra K, Olsen LJ, Theg SM (1989) Chloroplastic precursors and their transport across the envelope membranes. Annu Rev Plant Physiol Plant Mol Biol 40:471–501
Chua NH, Schmidt GW (1979) Transport of proteins into mitochondria and chloroplasts. J Cell Biol 81:461–483
Portis AR Jr, Salvucci ME, Ogren WL (1986) Activation of ribulosebisphosphate carboxylase/oxygenase at physiological CO2 and ribulosebisphosphate concentrations by Rubisco activase. Plant Physiol 82:967–971
Portis AR Jr (1995) The regulation of Rubisco by Rubisco activase. J Exp Bot 46:1285–1291
Jimenez ESD, Medrano L, Martinez-Barajas E (1995) Rubisco activae, a possible new member of the molecular chaperonefamily. Bichemistry 34:2826–2831
Han Y, Chen G, Wang Z (2000) The progress of studies on Rubisco activase. Chinese Bulletin of Botany 17(4):306–311. (in Chinese)
Robinson SP, Streusand VJ, Chatifield JM, Portis AR Jr (1988) Purification and assay of Rubisco activase from leaves. Plant Physiol 88:1008–1014
Lan Y, Mott KA (1991) Determination of apparent Km values for ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) activase using the spectrophotometric assay of Rubisco activity. Plant Physiol 95:604–609
Robert LH, Portis AR (2003) The life of ribulose-1,5-bisphosphate carboxylase/oxygenase-posttranslational facts and mysteries. Arch Biochem Biophys 414:150–158
Liu C, Hong FS, Wu K, Ma HB, Zhang XG, Hong CJ, Wu C, Gao FQ, Yang F, Zheng L, Wang XF, Liu T, Xie YN, Xu JH, Li ZR (2006) Effect of Nd3+ ion on carboxylation activity of ribulose-1,5-bisphosphate carboxylase/oxygenase of spinach. Biochemical and Biophysical Research Communications 342(1):36–43
Yang P, Lu C, Hua N, Du Y (2002) Titanim dioxide nanoparticles co-doped with Fe3+ and Eu3+ ions for photocatalysis. Mater Lett 57:794–801
Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24:1–15
Wang WG, Li LR (1980) A simplified purification method of RuBP carboxylase from spinach leaves. Acta Phytophysiologica Sinica 40(3):256–262. (in Chinese)
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Laboratory Press, NewYork, pp 739–750
Church GM, Gilbert W (1984) Genomic sequencing. Proc Natl Acad Sci U S A 8:1991–1995
Davis LG, Diber MD, Batter JF (1986) Basic methods in molecular biology. Elsevier, Paris
Sugiyama T, Nakayama N, Ogawa M, Akazawa T, Oda T (1986) Structure and function of chloroplast proteins: effect of ρ-chloromercuribenzoate treatment on the ribulose-1,5-bisphosphate carboxylase/oxygenase activity of Spinach leaf fraction protein. Arch Biochem Biophys 125:98–106
Salvucci ME, Klein RR (1994) Site-directed mutagenase of reaction lysyl residue(Lys-247) of Rubisco activase. Arch Biochem Biophys 314:178–185
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the folin phenol reagent. J Biol Chem 193:265–275
Tang RH, Jia JW, Li LR (1997) Purification and characterization of Rubisco activase from tobacco. Acta Phytophysiologica Sinica 23:89–95. (in Chinese)
Van de Loo FJ, Salvucci ME (1996) Activation of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) involves Rubisco activase Trp16. Biochemistry 35:8143–8148
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 277:680–685
To KY, Cheng MC, Chen LFO, Chen SCG (1996) Introduction and expression of foreign DNA in isolated spinach chloroplasts by electroporation. Plant J 10:737–743
Salvucci ME (1992) Subunit interactions of Rubisco activase–polyethylene glycol promotes self-association, stimulates ATPase and activation activities, and enhances interactions with Rubisco. Arch Biochem Biophys 298:688–696
To KY, Suen DF, Chen SCG (1999) Molecular characterization of ribulose-1,5-bisphosphate carboxylase/oxygenase activase in rice leaves. Planta 209:66–76
Zhang F, Jiang DA, Wen XY, Wang NY (2002) Changes in expression of rubisco activase from day to night in rice leave. Journal of Plant Physiology and Molecular Biology 28(1):37–40. (in Chinese)
Wayne MC, Anthony KB, David LO, Kenneth WJ (2004) Porphyrins as light harvesters in the dye-sensitised TiO2 solar cell. Coord Chem Rev 248:817–833
Abbott MS, Bogorad L (1987) Light regulation of genes for the large and small subunits of ribulose phosphate carboxylase in tobacco. Progress in Photosynthesis Research 4:527
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant no. 20671067), the Jiangsu Province Universities Natural Science Foundation (grant no. 06KJB180094), and the Medical Development Foundation of Suzhou University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Linglan, M., Chao, L., Chunxiang, Q. et al. Rubisco Activase mRNA Expression in Spinach: Modulation by Nanoanatase Treatment. Biol Trace Elem Res 122, 168–178 (2008). https://doi.org/10.1007/s12011-007-8069-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-007-8069-4