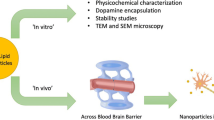
Abstract
In this work, nano-levodopa-liposomes (L-dopa-Lip) suspension was prepared by rotary-evaporated film-ultrasonic method, and freeze-drying powders of L-dopa-Lip were also obtained to improve the stability. The products were characterized by TEM, DLS, and TG-DSC, and the phase-transition temperature (Tm) and encapsulation efficiency were calculated. The brain-targeting and in vitro release of the drug was also studied. The results showed that L-dopa-Lip were well-formed spherical vesicles, and the sizes were about 100 nm, and the encapsulation efficiency was higher than 90%. The drug release temperature of L-dopa-Lip was 68 °C, and the in vitro release property and mathematical model were also studied. The brain targeting of L-dopa-Lip in vivo was explored by injecting the gold nanoparticles (AuNPs) labeled L-dopa-Lip (AuNPs-L-dopa-Lip) through the tail vein. ICP-MS and TEM showed that L-dopa-Lip had brain targeting, suggesting the potential treatment of L-dopa-Lip on brain dysfunction. The results of this work might be helpful for designing drug-loaded liposomes for the treatment of central nervous system (CNS) diseases and monitoring their distributions in vivo.








Similar content being viewed by others
Data Availability
All authors confirm that the data of this article is available inside the article. The datasets generated or analyzed during this study are available from the corresponding author upon reasonable request.
References
Fishbein, I., Kuo, Y. M., Giasson, B. I., & Nussbaum, R. L. (2014). Augmentation of phenotype in a transgenic Parkinson mouse heterozygous for a gaucher mutation. Brain, 137, 3235–3247. https://doi.org/10.1093/brain/awu291
Güneş, M., & Karavana, S. Y. (2022). Non-oral drug delivery in Parkinson’s Disease: Current applications and future. Turkish Journal of Pharmaceutical Sciences, 19, 343–352. https://doi.org/10.4274/tjps.galenos.2021.95226
Vázquez-Vélez, G. E., & Zoghbi, H. Y. (2021). Parkinson’s disease genetics and pathophysiology. Annual Review of Neuroscience, 44, 87–108. https://doi.org/10.1146/annurev-neuro-100720-034518
Oñate, M., Catenaccio, A., Salvadores, N., Saquel, C., Martinez, A., Moreno-Gonzalez, I., Gamez, N., Soto, P., Soto, C., Hetz, C., & Court, F. A. (2020). The necroptosis machinery mediates axonal degeneration in a model of Parkinson disease. Cell Death & Differentiation, 27, 1169–1185. https://doi.org/10.1038/s41418-019-0408-4
Weingarten, C. P., Sundman, M. H., Hickey, P., & Chen, N. K. (2015). Neuroimaging of Parkinson’s disease: Expanding views. Neuroscience & Biobehavioral Reviews, 59, 16–52. https://doi.org/10.1016/j.neubiorev.2015.09.007
Serra, P. A., Esposito, G., Enrico, P., Mura, M. A., Migheli, R., Delogu, M. R., Miele, M., Desole, M. S., Grella, G., & Miele, E. (2000). Manganese increases L-DOPA auto-oxidation in the striatum of the freely moving rat: Potential implications to L-DOPA long-term therapy of Parkinson’s disease. British Journal of Pharmacology, 130, 937–945. https://doi.org/10.1038/sj.bjp.0703379
Sampson, T. R., Debelius, J. W., Thron, T., Janssen, S., Shastri, G. G., Ilhan, Z. E., Challis, C., Schretter, C. E., Rocha, S., Gradinaru, V., Chesselet, M. F., Keshavarzian, A., Shannon, K. M., Krajmalnik-Brown, R., Wittung-Stafshede, P., Knight, R., & Mazmanian, S. K. (2016). Gut microbiota regulate motor deficits and neuroinflammation in a model of Parkinson’s disease. Cell, 167, 1469–1480. https://doi.org/10.1016/j.cell.2016.11.018
Rosenblad, C., Li, Q., Pioli, E. Y., Dovero, S., Antunes, A. S., Agúndez, L., Bardelli, M., Linden, R. M., Henckaerts, E., Björklund, A., Bezard, E., & Björklund, T. (2019). Vector-mediated l-3, 4-dihydroxyphenylalanine delivery reverses motor impairments in a primate model of Parkinson’s disease. Brain, 142, 2402–2416. https://doi.org/10.1093/brain/awz176
Fridjonsdottir, E., Shariatgorji, R., Nilsson, A., Vallianatou, T., Odell, L. R., Schembri, L. S., Svenningsson, P., Fernagut, P. O., Crossman, A. R., Bezard, E., & Andrén, P. E. (2021). Mass spectrometry imaging identifies abnormally elevated brain l-DOPA levels and extrastriatal monoaminergic dysregulation in l-DOPA-induced dyskinesia. Science Advances, 7, eabe5948. https://doi.org/10.1126/sciadv.abe5948
Cilia, R., Cereda, E., Akpalu, A., Sarfo, F. S., Cham, M., Laryea, R., Obese, V., Oppon, K., Del Sorbo, F., Bonvegna, S., Zecchinelli, A. L., & Pezzoli, G. (2020). Natural history of motor symptoms in Parkinson’s disease and the long-duration response to levodopa. Brain, 143, 2490–2501. https://doi.org/10.1093/brain/awaa181
Ford, G. A., Bhakta, B. B., Cozens, A., Hartley, S., Holloway, I., Meads, D., Pearn, J., Ruddock, S., Sackley, C. M., Saloniki, E. C., Santorelli, G., Walker, M. F., & Farrin, A. J. (2019). Safety and efficacy of co-careldopa as an add-on therapy to occupational and physical therapy in patients after stroke (DARS): A randomised, double-blind, placebo-controlled trial. The Lancet Neurology, 18, 530–538. https://doi.org/10.1016/S1474-4422(19)30147-4
Oldendorf, W. H. (1971). Brain uptake of radiolabeled amino acids, amines, and hexoses after arterial injection. American Journal of Physiology-Legacy Content, 221, 1629–1639. https://doi.org/10.1152/ajplegacy.1971.221.6.1629
Guo, X., Zheng, H., Guo, Y., Wang, Y., Anderson, G. J., Ci, Y., Yu, P., Geng, L., & Chang, Y. Z. (2017). Nasal delivery of nanoliposome-encapsulated ferric ammonium citrate can increase the iron content of rat brain. Journal of Nanobiotechnology, 15, 42. https://doi.org/10.1186/s12951-017-0277-2
Yuan, L., Geng, L., Ge, L., Yu, P., Duan, X., Chen, J., & Chang, Y. (2013). Effect of iron liposomes on anemia of inflammation. International Journal of Pharmaceutics, 454, 82–89. https://doi.org/10.1016/j.ijpharm.2013.06.078
Patil, Y. P., & Jadhav, S. (2014). Novel methods for liposome preparation. Chemistry and Physics of Lipids, 177, 8–18. https://doi.org/10.1016/j.chemphyslip.2013.10.011
Guan, J., Shen, Q., Zhang, Z., Jiang, Z., Yang, Y., Lou, M., Qian, J., Lu, W., & Zhan, C. (2018). Enhanced immunocompatibility of ligand-targeted liposomes by attenuating natural IgM absorption. Nature Communications, 9, 2982. https://doi.org/10.1038/s41467-018-05384-1
Neethirajan, S., & Jayas, D. S. (2011). Nanotechnology for the food and bioprocessing industries. Food and Bioprocess Technology, 4, 39–47. https://doi.org/10.1007/s11947-010-0328-2
Agrawal, M., Ajazuddin, Tripathi, D. K., Saraf, S., Saraf, S., Antimisiaris, S. G., Mourtas, S., Hammarlund-Udenaes, M., & Alexander, A. (2018). Recent advancements in liposomes targeting strategies to cross blood-brain barrier (BBB) for the treatment of Alzheimer’s disease. Journal of Controlled Release, 260, 61–77. https://doi.org/10.1016/j.jconrel.2017.05.019
Liu, X., Madhankumar, A. B., Miller, P. A., Duck, K. A., Hafenstein, S., Rizk, E., Slagle-Webb, B., Sheehan, J. M., Connor, J. R., & Yang, Q. X. (2016). MRI contrast agent for targeting glioma: interleukin-13 labeled liposome encapsulating gadolinium-DTPA. Neuro-Oncology, 18, 691–699. https://doi.org/10.1093/neuonc/nov263
Connor, E. E., Mwamuka, J., Gole, A., Murphy, C. J., & Wyatt, M. D. (2005). Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small (Weinheim An Der Bergstrasse, Germany), 1, 325–327. https://doi.org/10.1002/smll.200400093
Peng, J., & Liang, X. (2019). Progress in research on gold nanoparticles in cancer management. Medicine, 98, e15311. https://doi.org/10.1097/MD.0000000000015311
Lahiri, D., Nag, M., Dey, A., Sarkar, T., Pati, S., & Ray, R. R. (2022). Nanoparticles based antibacterial vaccines: Novel strategy to combat antimicrobial resistance. Process Biochemistry, 119, 82–89. https://doi.org/10.1016/j.procbio.2022.05.01
Bharadwaj, K. K., Rabha, B., Pati, S., Sarkar, T., Choudhury, K. B., Barman, A., Bhattacharjya, D., Srivastava, A., Baishya, D., Edinur, H. A., Kari, Z. A., & Noor, N. H. M. (2021). Green synthesis of gold nanoparticles using plant extracts as beneficial prospect for cancer theranostics. Molecules, 26, 6389. https://doi.org/10.3390/molecules26216389
Wang, H. H., Lin, C. A., Lee, C. H., Lin, Y. C., Tseng, Y. M., Hsieh, C. L., Chen, C. H., Tsai, C. H., Hsieh, C. T., Shen, J. L., Chan, W. H., Chang, W. H., & Yeh, H. I. (2011). Fluorescent gold nanoclusters as a biocompatible marker for in vitro and in vivo tracking of endothelial cells. ACS Nano, 5, 4337–4344. https://doi.org/10.1021/nn102752a
Lima, A. C., Campos, C. F., Cunha, C., Carvalho, A., Reis, R. L., Ferreira, H., & Neves, N. M. (2021). Biofunctionalized Liposomes to monitor rheumatoid arthritis regression stimulated by Interleukin-23 neutralization. Advanced Healthcare Materials, 10, e2001570. https://doi.org/10.1002/adhm.202001570
Ashton, J. R., Castle, K. D., Qi, Y., Kirsch, D. G., West, J. L., & Badea, C. T. (2018). Dual-energy CT imaging of tumor liposome delivery after gold nanoparticle-augmented radiation therapy. Theranostics, 8, 1782–1797. https://doi.org/10.7150/thno.22621
Rodrigues, A. R. O., Matos, J. O. G., Dias, N., Almeida, A. M., Pires, B. G., Pereira, A., Araújo, A. M., Queiroz, J. P., Castanheira, M. R. P., E. M. S., & Coutinho, P. J. G. (2018). Development of multifunctional liposomes containing magnetic/plasmonic MnFe2O4/Au core / shell nanoparticles. Pharmaceutics, 11, 10. https://doi.org/10.3390/pharmaceutics11010010
Prasad, R., Jain, N. K., Yadav, A. S., Chauhan, D. S., Devrukhkar, J., Kumawat, M. K., Shinde, S., Gorain, M., Thakor, A. S., Kundu, G. C., Conde, J., & Srivastava, R. (2020). Liposomal nanotheranostics for multimode targeted in vivo bioimaging and near-infrared light mediated cancer therapy. Communications Biology, 3, 284. https://doi.org/10.1038/s42003-020-1016-z
Wang, S., Li, W., Sun, K., Zhang, R., Wang, S., & Geng, L. (2019). Study of release kinetics and degradation thermodynamics of ferric citrate liposomes. Chemistry and Physics of Lipids, 225, 104811. https://doi.org/10.1016/j.chemphyslip.2019.104811
Chen, C., Han, D., Cai, C., & Tang, X. (2010). An overview of liposome lyophilization and its future potential. Journal of Controlled Release, 142, 299–311. https://doi.org/10.1016/j.jconrel.2009.10.024
Limasale, Y. D., Tezcaner, A., Özen, C., Keskin, D., & Banerjee, S. (2015). Epidermal growth factor receptor-targeted immunoliposomes for delivery of celecoxib to cancer cells. International Journal of Pharmaceutics, 479, 364–373. https://doi.org/10.1016/j.ijpharm.2015.01.016
Soni, V., Kohli, D. V., & Jain, S. K. (2008). Transferrin coupled liposomes as drug delivery carriers for brain targeting of 5-florouracil. Journal of Drug Targeting, 16, 73–78. https://doi.org/10.1080/10611860701725381
Wen, C. J., Zhang, L. W., Al-Suwayeh, S. A., Yen, T. C., & Fang, J. Y. (2012). Theranostic liposomes loaded with quantum dots and apomorphine for brain targeting and bioimaging. International Journal of Nanomedicine, 7, 1599–1611. https://doi.org/10.2147/IJN.S29369
Funding
This work is completed with funding from the National Natural Science Foundation of China (31201305), the Natural Science Foundation of Hebei Province (B2019205054, B2021205001, E2022205011), the Science Foundation of Hebei Normal University (L2023J01), and the Teaching Reform project of Hebei Normal University (2021XJJG047).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Lina Geng and Yayong Li designed the research, material preparation, and characterization carried out by Yue Hu, and the in vivo experiments were tested by Lin Wang and Sha Liu. The first draft of the manuscript was written by Shenna Chen, and the check of the manuscript was done by Lina Geng and Yayong Li. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical Approval
This study and included experimental procedures were approved by the Laboratory Animal Ethical and Welfare Committee (AEWC) of Hebei Normal University (IACUC-167006). All animal housing and experiments were conducted in strict accordance with the institutional guidelines for the care and use of laboratory animals.
Consent to Participate
All authors agree to participate in this work, and they affirm that it is original research.
Consent for Publication
All authors have their consent to publish their work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 670 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, S., Wang, L., Hu, Y. et al. High Drug Capacity of Nano-Levodopa-Liposomes: Preparation, In Vitro Release and Brain-Targeted Research. Appl Biochem Biotechnol (2023). https://doi.org/10.1007/s12010-023-04673-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s12010-023-04673-w