Abstract
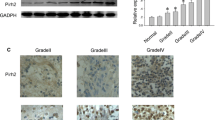
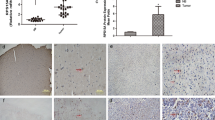
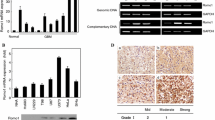
Accumulating evidence indicates Ribosomal protein 34 (RPL34) promotes tumor malignance and its expression is associated with poor prognosis in multiple cancer cells. However, the physiological role and biological mechanism of RPL34 in glioblastoma (GBM) remain unclear. Hence, this study aimed to investigate the expression and the role of RPL34 in GBM. A total of 59 glioma samples and 12 normal brains for epilepsy surgery were used to determine the underlying mechanisms and the biological behaviors of RPL34 in GBM. In this study, we identified that RPL34 expression was significantly (p < 0.05) enriched in GBM tumors compared with low-grade glioma and normal brain, and its expression was associated with poor survival. Additionally, RPL34 was functionally required for tumor proliferation in vitro. Mechanically, inhibition of RPL34 induced glioma cell apoptosis by activation of Bad/Caspase7/PARP signaling pathway. The RPL34 promotes cell survival in GBM and could be a potential therapeutic target for GBM.





Similar content being viewed by others
Data Availability
Not applicable.
References
Stupp, R., Mason, W. P., van den Bent, M. J., Weller, M., Fisher, B., Taphoorn, M. J., et al. (2005). Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. New England Journal of Medicine, 352, 987–996.
Guarente, V., & Sportoletti, P. (2021). Lessons, Challenges and future therapeutic opportunities for PI3K inhibition in CLL. Cancers, 13, 280.
Herbst, R. S., Morgensztern, D., & Boshoff, C. (2018). The biology and management of non-small cell lung cancer. Nature, 553, 446–454.
Higgins, M. J., & Baselga, J. (2011). Targeted therapies for breast cancer. The Journal of Clinical Investigation, 121, 3797–3803.
Herbst, R. S., Soria, J. C., Kowanetz, M., Fine, G. D., & Hamid, O. (2014). Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature, 27, 563–567.
Phan, G. Q., Yang, J. C., Sherry, R. M., Hwu, P., Topalian, S. L., Schwartzentruber, D. J., et al. (2003). Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proceedings of the National Academy of Sciences of the United States of America, 8, 8372–9377.
Tumeh, P. C., Harview, C. L., Yearley, J. H., Shintaku, I. P., Taylor, E. J., Robert, L., et al. (2014). PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature, 27, 568–571.
Zhang, Y., & Lu, H. (2009). Signaling to p53: Ribosomal proteins find their way. Cancer Cell, 16, 369–377.
Pelletier, J., Thomas, G., & Volarevic, S. (2018). Ribosome biogenesis in cancer: New players and therapeutic avenues. Nature Reviews Cancer, 18, 51–63.
Nissan, T. A., Bassler, J., Petfalski, E., Tollervey, D., & Hurt, E. (2002). 60S pre-ribosome formation viewed from assembly in the nucleolus until export to the cytoplasm. EMBO Journal, 21, 5539–5547.
Ji, P., Wang, L., Liu, J., Mao, P., Li, R., Jiang, H., et al. (2019). Knockdown of RPL34 inhibits the proliferation and migration of glioma cells through the inactivation of JAK/STAT3 signaling pathway. Journal of Cellular Biochemistry, 120, 3259–3267.
Fan, H., Li, J., Jia, Y., Wu, J., Yuan, L., Li, M., et al. (2017). Silencing of ribosomal protein L34 (RPL34) inhibits the proliferation and invasion of esophageal cancer cells. Oncology Research, 7, 1061–1068.
Dai, J., & Wei, W. (2017). Influence of the RPL34 gene on the growth and metastasis of oral squamous cell carcinoma cells. Archives of Oral Biology, 83, 40–46.
Wei, F., Ding, L., Wei, Z., Zhang, Y., Li, Y., Qinghua, L., et al. (2016). Ribosomal protein L34 promotes the proliferation, invasion and metastasis of pancreatic cancer cells. Oncotarget, 7(51), 85259.
Luo, S., Zhao, J., Fowdur, M., Wang, K., Jiang, T., & He, M. (2016). Highly expressed ribosomal protein L34 indicates poor prognosis in osteosarcoma and its knockdown suppresses osteosarcoma proliferation probably through translational control. Scientific Reports, 6(1), 1–4.
Yang, S., Cui, J., Yang, Y., Liu, Z., Yan, H., Tang, C., et al. (2016). Over-expressed RPL34 promotes malignant proliferation of non-small cell lung cancer cells. Gene, 576, 421–428.
Liu, H., Liang, S., Yang, X., Ji, Z., Zhao, W., Ye, X., et al. (2015). RNAi-mediated RPL34 knockdown suppresses the growth of human gastric cancer cells. Oncology Reports, 34, 2267–2272.
Van den Haute, C., Eggermont, K., Nuttin, B., Debyser, Z., & Baekelandt, V. (2003). Lentiviral vector-mediated delivery of short hairpin RNA results in persistent knockdown of gene expression in mouse brain. Human Gene Therapy, 14(18), 1799–1807.
van Tonder, A., Joubert, A. M., & Cromarty, A. D. (2015). Limitations of the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assay when compared to three commonly used cell enumeration assays. BMC Research Notes, 20(8), 47. https://doi.org/10.1186/s13104-015-1000-8.PMID:25884200;PMCID:PMC4349615
Rosenquist, M. (2003). 14–3–3 proteins in apoptosis. Brazilian Journal of Medical and Biological Research, 36, 403–408.
Sastry, K. S., Karpova, Y., & Kulik, G. (2006). Epidermal growth factor protects prostate cancer cells from apoptosis by inducing BAD phosphorylation via redundant signaling pathways. Journal of Biological Chemistry, 281, 27367–27377.
Zha, J., Harada, H., Yang, E., Jockel, J., & Korsmeyer, S. J. (1996). Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X(L). Cell, 15, 619–628.
Datta, S. R., Ranger, A. M., Lin, M. Z., Sturgill, J. F., Ma, Y. C., Cowan, C. W., et al. (2002). Survival factor-mediated BAD phosphorylation raises the mitochondrial threshold for apoptosis. Developmental Cell, 3(5), 631–643.
Boucher, D., Blais, V., & Denault, H. B. (2012). Caspase-7 uses an exosite to promote poly(ADP ribose) polymerase 1 proteolysis. Proceedings of the National academy of Sciences of the United States of America, 109, 5669–5674.
Rodríguez-Hernández, A., Brea-Calvo, G., Fernández-Ayala, D. J., Cordero, M., & Navas, P. (2006). Nuclear caspase-3 and caspase-7 activation, and poly (ADP-ribose) polymerase cleavage are early events in camptothecin-induced apoptosis. Apoptosis, 11, 131–139.
Harada, H., Andersen, J. S., Mann, M., Terada, N., & Korsmeyer, S. J. (2001). p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proceedings of the National academy of Sciences of the United States of America, 14, 9666–9670.
Rathinaswamy, M. K., & Burke, J. E. (2020). Class I phosphoinositide 3-kinase (PI3K) regulatory subunits and their roles in signaling and disease. Advances in Biological Regulation, 75, 100657.
Funding
This work was supported by the Natural Science Basic Research Plan in Shaanxi Province of China, Grant/Award (No.2019JM-498), the Clinical Research Award (No. XJTU1AF-CRF-2016–018), and the Special Foundation for “Class A Subject” of the First Affiliated Hospital of Xi’an Jiaotong University, China.
Author information
Authors and Affiliations
Contributions
CWD, LNS, and MDW contributed to the study concept and design. CWD and LNS contributed to the major research work and data collection. ZD contributed to IHC staining. MW, JJL, YX, and NW contributed to glioma samples and clinical information collection. CWD, JW, and PM contributed to data analysis. CWD wrote the manuscript. CW, HTJ, JW, and MDW contributed to critical revision. All the authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
Not applicable.
Consent to Participate
All authors have their consent to participate.
Consent to Publish
All authors have their consent to publish their work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Du, C., Wang, T., Jia, J. et al. Suppression of RPL34 Inhibits Tumor Cell Proliferation and Promotes Apoptosis in Glioblastoma. Appl Biochem Biotechnol 194, 3494–3506 (2022). https://doi.org/10.1007/s12010-022-03857-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03857-0