Abstract
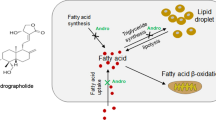
Inhibition of lipid accumulation is the key step to prevent nonalcoholic fatty liver (NAFL) progressing to nonalcoholic steatohepatitis. We aimed to study the effect of low-molecular-weight citrus pectin (LCP) against lipid accumulation and the underlying mechanism. Oleic acid (OA)-induced lipid deposition in HepG2 cells was applied to mimic in vitro model of lipid accumulation. Oil Red O (ORO) stain result showed lipid accumulation was significantly reduced, and levels of adipose triglyceride lipase (ATGL) and carnitine palmitoyltransferase-1 (CPT-1), involved in triacylglycerol catabolism and fatty acid β-oxidation, detected by RT-qPCR were increased after OA-stimulated HepG2 cells treated with LCP. RNA sequencing analysis identified 740 differentially expressed genes (DEGs) in OA-stimulated HepG2 cells treated with the LCP group (OA+LCP group), and bioinformatics analysis indicated that some DEGs were enriched in lipid metabolism-related processes and pathways. The expression of the top 8 known DEGs in the OA+LCP group was then verified by RT-qPCR, which showed that fold change (abs) of METTL7B was the highest among the 8 candidates. In addition, overexpression of METTL7B in HepG2 cells significantly inhibited the lipid accumulation and enhanced levels of ATGL and CPT-1. In conclusion, LCP inhibited lipid accumulation through the upregulation of METTL7B, and further enhancement of ATGL and CPT-1 levels. LCP is expected to develop as a promising agent to ameliorate fat accumulation in NAFL.




Similar content being viewed by others
References
Sheka, A. C., Adeyi, O., Thompson, J., Hameed, B., Crawford, P. A., & Ikramuddin, S. (2020). Nonalcoholic steatohepatitis: a review. JAMA, 323(12), 1175–1183.
Dietrich, P., & Hellerbrand, C. (2014). Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Practice & Research. Clinical Gastroenterology, 28(4), 637–653.
Dibba, P., Li, A., Perumpail, B., John, N., Sallam, S., Shah, N., et al. (2018). Emerging therapeutic targets and experimental drugs for the treatment of NAFLD. Diseases, 6(3), 1–15.
Younossi, Z. M., Ratziu, V., Loomba, R., Rinella, M., Anstee, Q. M., Goodman, Z., Bedossa, P., Geier, A., Beckebaum, S., Newsome, P. N., Sheridan, D., Sheikh, M. Y., Trotter, J., Knapple, W., Lawitz, E., Abdelmalek, M. F., Kowdley, K. V., Montano-Loza, A. J., Boursier, J., Mathurin, P., Bugianesi, E., Mazzella, G., Olveira, A., Cortez-Pinto, H., Graupera, I., Orr, D., Gluud, L. L., Dufour, J. F., Shapiro, D., Campagna, J., Zaru, L., MacConell, L., Shringarpure, R., Harrison, S., Sanyal, A. J., Abdelmalek, M., Abrams, G., Aguilar, H., Ahmed, A., Aigner, E., Aithal, G., Ala, A., Alazawi, W., Albillos, A., Allison, M., al-Shamma, S., Andrade, R., Andreone, P., Angelico, M., Ankoma-Sey, V., Anstee, Q., Anty, R., Araya, V., Arenas Ruiz, J. I., Arkkila, P., Arora, M., Asselah, T., Au, J., Ayonrinde, O., Bailey, R. J., Balakrishnan, M., Bambha, K., Bansal, M., Barritt, S., Bate, J., Beato, J., Beckebaum, S., Behari, J., Bellot, P., Ben Ari, Z., Bennett, M., Berenguer, M., Beretta-Piccoli, B. T., Berg, T., Bonacini, M., Bonet, L., Borg, B., Bourliere, M., Boursier, J., Bowman, W., Bradley, D., Brankovic, M., Braun, M., Bronowicki, J. P., Bruno, S., Bugianesi, E., Cai, C., Calleja Panero, J. L., Carey, E., Carmiel, M., Carrión, J. A., Cave, M., Chagas, C., Chami, T., Chang, A., Coates, A., Cobbold, J., Corey, K., Corless, L., Cortez-Pinto, H., Crespo, J., Cruz Pereira, O., de Ledinghen, V., deLemos, A., Diago, M., Dufour, J. F., Dugalic, P., Dunn, W., Elkhashab, M., Epstein, M., Escudero-Garcia, M. D., Etzion, O., Evans, L., Falcone, R., Fernandez, C., Ferreira, J., Fink, S., Finnegan, K., Firpi-Morell, R., Floreani, A., Fontanges, T., Ford, R., Forrest, E., Fowell, A., Fracanzani, A. L., Francque, S., Freilich, B., Frias, J., Fuchs, M., Fuentes, J., Galambos, M., Gallegos, J., Geerts, A., Geier, A., George, J., Ghali, M., Ghalib, R., Gholam, P., Gines, P., Gitlin, N., Gluud, L. L., Goeser, T., Goff, J., Gordon, S., Gordon, F., Goria, O., Greer, S., Grigorian, A., Gronbaek, H., Guillaume, M., Gunaratnam, N., Halegoua-de Marzio, D., Hameed, B., Hametner, S., Hamilton, J., Harrison, S., Hartleb, M., Hassanein, T., Häussinger, D., Hellstern, P., Herring, R., Heurich, E., Hezode, C., Hinrichsen, H., Holland Fischer, P., Horsmans, Y., Huang, J., Jakiche, A., Jeffers, L., Jones, B., Jorge, R., Jorquera, F., Kahraman, A., Kaita, K., Karyotakis, N., Kayali, Z., Kechagias, S., Kepczyk, T., Khalili, M., Khallafi, H., Kluwe, J., Knapple, W., Kohli, A., Korenblat, K., Kowdley, K., Krag, A., Krause, R., Kremer, A., Krok, K., Krstic, M., Kugelmas, M., Kumar, S., Labarriere, D., Lai, M., Lampertico, P., Lawitz, E., Lee, A., Leroy, V., Lidofsky, S., Lim, T. H., Lim, J., Lipkis, D., Little, E., Lonardo, A., Long, M., Loomba, R., Lurie, Y., Macedo, G., Makara, M., Maliakkal, B., Manns, M., Manousou, P., Mantry, P., Marchesini, G., Marinho, C., Marotta, P., Marschall, H. U., Mathurin, P., Mayo, M., Mazzella, G., McCullen, M., McLaughlin, W., Merriman, R., Modi, A., Molina, E., Montano-Loza, A., Monteverde, C., Moreea, S., Moreno, C., Morisco, F., Mubarak, A., Muellhaupt, B., Mukherjee, S., Müller, T., Nagorni, A., Naik, J., Neff, G., Nevah, M., Newsome, P., Nguyen-Khac, E., Noureddin, M., Oben, J., Olveira, A., Orlent, H., Orr, D., Orr, J., Ortiz-Lasanta, G., Ozenne, V., Pandya, P., Paredes, A., Park, J., Patel, J., Patel, K., Uta, M., Patton, H., Peck-Radosavljevic, M., Petta, S., Pianko, S., Piekarska, A., Pimstone, N., Pockros, P., Pol, S., Porayko, M., Poulos, J., Pound, D., Pouzar, J., Presa Ramos, J., Pyrsopoulos, N., Rafiq, N., Muller, K., Ramji, A., Ratziu, V., Ravinuthala, R., Reddy, C., Reddy K G, G., Reddy K R, K. R., Regenstein, F., Reindollar, R., Riera, A., Rinella, M., Rivera Acosta, J., Robaeys, G., Roberts, S., Rodriguez-Perez, F., Romero-Gomez, M., Rubin, R., Rumi, M., Rushbrook, S., Rust, C., Ryan, M., Safadi, R., Said, A., Salminen, K., Samuel, D., Santoro, J., Sanyal, A., Sarkar, S., Schaeffer, C., Schattenberg, J., Schiefke, I., Schiff, E., Schmidt, W., Schneider, J., Schouten, J., Schultz, M., Sebastiani, G., Semela, D., Sepe, T., Sheikh, A., Sheikh, M., Sheridan, D., Sherman, K., Shibolet, O., Shiffman, M., Siddique, A., Sieberhagen, C., Sigal, S., Sikorska, K., Simon, K., Sinclair, M., Skoien, R., Solis, J., Sood, S., Souder, B., Spivey, J., Stal, P., Stinton, L., Strasser, S., Svorcan, P., Szabo, G., Talal, A., Tam, E., Tetri, B., Thuluvath, P., Tobias, H., Tomasiewicz, K., Torres, D., Trauner, M., Trautwein, C., Trotter, J., Tsochatzis, E., Unitt, E., Vargas, V., Varkonyi, I., Veitsman, E., Vespasiani Gentilucci, U., Victor, D., Vierling, J., Vincent, C., Vincze, A., von der Ohe, M., von Roenn, N., Vuppalanchi, R., Waters, M., Watt, K., Weltman, M., Wieland, A., Wiener, G., Williams A, A., Williams J, J., Wilson, J., Yataco, M., Yoshida, E., Younes, Z., Yuan, L., Zivony, A., Zogg, D., Zoller, H., Zoulim, F., Zuckerman, E., & Zuin, M. (2019). Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet, 394(10215), 2184–2196.
Harrison, S. A., Bashir, M. R., Guy, C. D., Zhou, R., Moylan, C. A., Frias, J. P., Alkhouri, N., Bansal, M. B., Baum, S., Neuschwander-Tetri, B. A., Taub, R., & Moussa, S. E. (2019). Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet, 394(10213), 2012–2024.
Chen, Y., Feng, R., Yang, X., Dai, J., Huang, M., Ji, X., Li, Y., Okekunle, A. P., Gao, G., Onwuka, J. U., Pang, X., Wang, C., Li, C., Li, Y., & Sun, C. (2019). Yogurt improves insulin resistance and liver fat in obese women with nonalcoholic fatty liver disease and metabolic syndrome: a randomized controlled trial. The American Journal of Clinical Nutrition, 109(6), 1611–1619.
Ciriminna, R., Fidalgo, A., Delisi, R., Tamburino, A., Carnaroglio, D., Cravotto, G., Ilharco, L. M., & Pagliaro, M. (2017). Controlling the degree of esterification of citrus pectin for demanding applications by selection of the source. ACS Omega, 2(11), 7991–7995.
Delphi, L., & Sepehri, H. (2016). Apple pectin: a natural source for cancer suppression in 4T1 breast cancer cells in vitro and express p53 in mouse bearing 4T1 cancer tumors, in vivo. Biomedicine & Pharmacotherapy, 84, 637–644.
Wang, S., Li, P., Lu, S. M., & Ling, Z. Q. (2016). Chemoprevention of low-molecular-weight citrus pectin (LCP) in gastrointestinal cancer cells. International Journal of Biological Sciences, 12(6), 746–756.
Eliaz, I., & Raz, A. (2019). Pleiotropic effects of modified citrus pectin. Nutrients, 11(11), 1–18.
Abu-Elsaad, N. M., & Elkashef, W. F. (2016). Modified citrus pectin stops progression of liver fibrosis by inhibiting galectin-3 and inducing apoptosis of stellate cells. Canadian Journal of Physiology and Pharmacology, 94(5), 554–562.
Martinez-Martinez, E., Calvier, L., Rossignol, P., Rousseau, E., Fernandez-Celis, A., Jurado-Lopez, R., Laville, M., Cachofeiro, V., & Lopez-Andres, N. (2016). Galectin-3 inhibition prevents adipose tissue remodelling in obesity. International Journal of Obesity, 40(6), 1034–1038.
Marin-Royo, G., et al. (2018). Inhibition of galectin-3 ameliorates the consequences of cardiac lipotoxicity in a rat model of diet-induced obesity. Disease Models & Mechanisms, 11(2), dmm032086.
Eliaz, I., Hotchkiss, A. T., Fishman, M. L., & Rode, D. (2006). The effect of modified citrus pectin on urinary excretion of toxic elements. Phytotherapy Research, 20(10), 859–864.
Hong, Y., Choi, S. I., Hong, E., & Kim, G. H. (2020). Psoralea corylifolia L. extract ameliorates nonalcoholic fatty liver disease in free-fatty-acid-incubated HEPG2 cells and in high-fat diet-fed mice. Journal of Food Science, 85(7), 2216–2226.
Guo, L., Kang, J. S., Park, Y. H., Je, B. I., Lee, Y. J., Kang, N. J., Park, S. Y., Hwang, D. Y., & Choi, Y. W. (2020). S-petasin inhibits lipid accumulation in oleic acid-induced HepG2 cells through activation of the AMPK signaling pathway. Food & Function, 11(6), 5664–5673.
Ren, G., Guo, J. H., Qian, Y. Z., Kong, W. J., & Jiang, J. D. (2020). Berberine improves glucose and lipid metabolism in HepG2 cells through AMPKalpha1 activation. Frontiers in Pharmacology, 11, 647.
Yang, J., et al. (2013). Glucagon-like peptide 1 regulates adipogenesis in 3T3-L1 preadipocytes. International Journal of Molecular Medicine, 31(6), 1429–1435.
Livak, K. J., & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods, 25(4), 402–408.
European Association for the Study of the, L., European Association for the Study of, D., & European Association for the Study of, O. (2016). EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Obesity Facts, 9(2), 65–90.
Chalasani, N., Younossi, Z., Lavine, J. E., Diehl, A. M., Brunt, E. M., Cusi, K., Charlton, M., & Sanyal, A. J. (2012). The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology, 55(6), 2005–2023.
Liu, Y. T., Lai, Y. H., Lin, H. H., & Chen, J. H. (2019). Lotus seedpod extracts reduced lipid accumulation and lipotoxicity in hepatocytes. Nutrients, 11(12), 1–15.
Zhang, J., Zhang, S. D., Wang, P., Guo, N., Wang, W., Yao, L. P., Yang, Q., Efferth, T., Jiao, J., & Fu, Y. J. (2019). Pinolenic acid ameliorates oleic acid-induced lipogenesis and oxidative stress via AMPK/SIRT1 signaling pathway in HepG2 cells. European Journal of Pharmacology, 861, 172618.
Shen, B., Feng, H., Cheng, J., Li, Z., Jin, M., Zhao, L., Wang, Q., Qin, H., & Liu, G. (2020). Geniposide alleviates non-alcohol fatty liver disease via regulating Nrf2/AMPK/mTOR signalling pathways. Journal of Cellular and Molecular Medicine, 24(9), 5097–5108.
Mun, J., et al. (2019). Water extract of Curcuma longa L. ameliorates non-alcoholic fatty liver disease. Nutrients, 11(10), 1–13.
Zimmermann, R., Strauss, J. G., Haemmerle, G., Schoiswohl, G., Birner-Gruenberger, R., Riederer, M., Lass, A., Neuberger, G., Eisenhaber, F., Hermetter, A., & Zechner, R. (2004). Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science, 306(5700), 1383–1386.
Wang, Y., Chen, Y., Guan, L., Zhang, H., Huang, Y., Johnson, C. H., Wu, Z., Gonzalez, F. J., Yu, A., Huang, P., Wang, Y., Yang, S., Chen, P., Fan, X., Huang, M., & Bi, H. (2018). Carnitine palmitoyltransferase 1C regulates cancer cell senescence through mitochondria-associated metabolic reprograming. Cell Death and Differentiation, 25(4), 735–748.
Pramfalk, C., et al. (2020). Generation of new hepatocyte-like in vitro models better resembling human lipid metabolism. Biochimica et Biophysica Acta - Molecular and Cell Biology of Lipids, 1865(6), 158659.
Sodhi, S. S., Ghosh, M., Song, K. D., Sharma, N., Kim, J. H., Kim, N. E., Lee, S. J., Kang, C. W., Oh, S. J., & Jeong, D. K. (2014). An approach to identify SNPs in the gene encoding acetyl-CoA acetyltransferase-2 (ACAT-2) and their proposed role in metabolic processes in pig. PLoS One, 9(7), e102432.
Korner, A., et al. (2019). Inhibition of delta24-dehydrocholesterol reductase activates pro-resolving lipid mediator biosynthesis and inflammation resolution. Proceedings of the National Academy of Sciences of the United States of America, 116(41), 20623–20634.
Wu, X., Sang, L., & Gong, Y. (2018). N6-methyladenine RNA modification and cancers. American Journal of Cancer Research, 8(10), 1957–1966.
Chen, M., & Wong, C. M. (2020). The emerging roles of N6-methyladenosine (m6A) deregulation in liver carcinogenesis. Molecular Cancer, 19(1), 44.
Zhong, H., Tang, H. F., & Kai, Y. (2020). N6-methyladenine RNA modification (m6A): an emerging regulator of metabolic diseases. Current Drug Targets, 21(11), 1056–1067.
Luo, Z., Zhang, Z., Tai, L., Zhang, L., Sun, Z., & Zhou, L. (2019). Comprehensive analysis of differences of N(6)-methyladenosine RNA methylomes between high-fat-fed and normal mouse livers. Epigenomics, 11(11), 1267–1282.
Lu, N., Li, X., Yu, J., Li, Y., Wang, C., Zhang, L., Wang, T., & Zhong, X. (2018). Curcumin attenuates lipopolysaccharide-induced hepatic lipid metabolism disorder by modification of m(6) A RNA methylation in piglets. Lipids, 53(1), 53–63.
Zhong, X., Yu, J., Frazier, K., Weng, X., Li, Y., Cham, C. M., Dolan, K., Zhu, X., Hubert, N., Tao, Y., Lin, F., Martinez-Guryn, K., Huang, Y., Wang, T., Liu, J., He, C., Chang, E. B., & Leone, V. (2018). Circadian clock regulation of hepatic lipid metabolism by modulation of m(6) A mRNA methylation. Cell Reports, 25(7), 1816–1828 e1814.
Kang, H., Zhang, Z., Yu, L., Li, Y., Liang, M., & Zhou, L. (2018). FTO reduces mitochondria and promotes hepatic fat accumulation through RNA demethylation. Journal of Cellular Biochemistry, 119(7), 5676–5685.
Funding
This work was supported by the Natural Science Research Project of Shanghai Minhang Science and Technology Committee (2018MHZ074, 2019MHZ069) and Special Construction Project of Integrated Traditional Chinese and Western Medicine in Shanghai General Hospital (ZHYY-ZXYJHZX-201622).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, X., Yuan, Y. & Xie, D. Low Molecular Pectin Inhibited the Lipid Accumulation by Upregulation of METTL7B. Appl Biochem Biotechnol 193, 1469–1481 (2021). https://doi.org/10.1007/s12010-021-03486-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-021-03486-z