Abstract
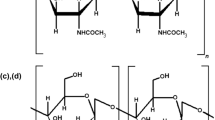
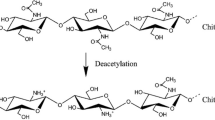
The natural biopolymer chitin and its deacetylated product chitosan are found abundantly in nature as structural building blocks and are used in all sectors of human activities like materials science, nutrition, health care, and energy. Far from being fully recognized, these polymers are able to open opportunities for completely novel applications due to their exceptional properties which an economic value is intrinsically entrapped. On a commercial scale, chitosan is mainly obtained from crustacean shells rather than from the fungal and insect sources. Significant efforts have been devoted to commercialize chitosan extracted from fungal and insect sources to completely replace crustacean-derived chitosan. However, the traditional chitin extraction processes are laden with many disadvantages. The present review discusses the potential bioextraction of chitosan from fungal, insect, and crustacean as well as its superior physico-chemical properties. The different aspects of fungal, insects, and crustacean chitosan extraction methods and various parameters having an effect on the yield of chitin and chitosan are discussed in detail. In addition, this review also deals with essential attributes of chitosan for high value-added applications in different fields and highlighted new perspectives on the production of chitin and deacetylated chitosan from different sources with the concomitant reduction of the environmental impact.




Similar content being viewed by others
References
Abdel-Rahman, R. M., Hrdina, R., Abdel-Mohsen, A. M., Fouda, M. M., Soliman, A. Y., Mohamed, F. K., & Mohsin, K. (2015). Chitin and chitosan from Brazilian Atlantic Coast: isolation, characterization and antibacterial activity. International Journal of Biological Macromolecules, 80, 107–120.
Aguila, E., Gomes, L., Andrade, C., Silva, J., & Paschoalin, V. (2012). Biocatalytic production of chitosan polymers from shrimp shells, using a recombinant enzyme produced by Pichia pastoris. American Journal of Molecular Biology, 2, 341–350.
Alireza, T., Gorji, A., Kalhor, H., Sajadian, A., Tabarraei, A., Moradi, A., Atyabi, F., & Kelishadi, M. (2014) Antitumor effect of therapeutic HPV DNA vaccines with chitosan-based nanodelivery systems. Biomedical Science, 1–10.
Andrade, S. M. B. d., Ladchumananandasivam, R., Rocha, B. G. d., Belarmino, D. D., & Galvão, A. O. (2012). The use of exoskeletons of shrimp (Litopenaeus vanammei) and crab (Ucides cordatus) for the extraction of chitosan and production of Nanomembrane. Materials Sciences and Applications, 3, 495–508.
Aranaz, I., Mengíbar, M., Harris, R., Paños, I., Miralles, B., Acosta, M., et al. (2009). Functional characterization of chitin and chitosan. Current Chemical Biology, 3, 203–230.
Arbia, W., Adour, L., Amrane, A., & Lounici, H. (2013). Optimization of medium composition for enhanced chitin extraction from Parapenaeus Longirostris by Lactobacillus helveticus using response surface methodology. Food Hydrocolloids, 31, 392–403.
Aytekin, O., & Elibol, M. (2010). Cocultivation of Lactococcus lactis and Teredinobacter turnirae for biological chitin extraction from prawn waste. Bioprocess and Biosystems Engineering, 33, 393–399.
Badawy, R., & Mohamed, H. (2015). Chitin extration, composition of different six insect species and their comparable characteristics with that of the shrimp. Journal of American Science, 11, 127–134.
Bajaj, M., Winter, J., & Gallert, C. (2011). Effect of deproteination and deacetylation conditions on viscosity of chitin and chitosan extracted from Crangon crangon shrimp waste. Biochemical Engineering Journal, 56, 51–62.
Beier, S., & Bertilsson, S. (2013). Bacterial chitin degradation-mechanisms and ecophysiological strategies. Frontiers in Microbiology, 4, 1–12.
Bendig, C., Kraxenberger, T., & Römer, L. (2013). Shaping the future with industrial biotechnology new and efficient production processes for biopolymers. JSM Biotechnology Bioengineering, 2, 1–9.
Benhabiles, M. S., Abdi, N., Drouiche, N., Lounici, H., Pauss, A., Goosen, M. F. A., & Mameri, N. (2013). Protein recovery by ultrafiltration during isolation of chitin from shrimp shells Parapenaeus longirostris. Food Hydrocolloids, 32, 28–34.
Benhabiles, M. S., Drouiche, N., Lounici, H., Pauss, A., & Mameri, N. (2013). Effect of shrimp chitosan coatings as affected by chitosan extraction processes on postharvest quality of strawberry. Journal of Food Measurement and Characterization, 7, 215–221.
Benhabiles, M. S., Salah, R., Lounici, H., Drouiche, N., Goosen, M. F. A., & Mameri, N. (2012). Antibacterial activity of chitin, chitosan and its oligomers prepared from shrimp shell waste. Food Hydrocolloids, 29, 48–56.
Benhabiles, M. S., Tazdait, D., Abdi, N., Lounici, H., Drouiche, N., Goosen, M. F. A., & Mameri, N. (2013). Assessment of coating tomato fruit with shrimp shell chitosan and N,O-carboxymethyl chitosan on postharvest preservation. Journal of Food Measurement and Characterization, 7, 66–74.
Berger, L., Stamford, N. P., Willadino, L. G., Laranjeira, D., de Lima, M. A., Malheiros, S., & de Oliveira, W. J. (2016). Cowpea resistance induced against fusarium oxysporum f. Sp. tracheiphilum by crustaceous chitosan and by biomass and chitosan obtained from Cunninghamella elegans. Biological Control, 92, 45–54.
Berger, L. R., Stamford, T. C., Stamford-Arnaud, T. M., de Alcantara, S. R., da Silva, A. M., do Nascimento, A. E., & de Campos-Takaki, G. M. (2014). Green conversion of agroindustrial wastes into chitin and chitosan by Rhizopus arrhizus and Cunninghamella Elegans strains. International Journal of Molecular Sciences, 15, 9082–9102.
Bijay, S., Choi, Y., Akaike, T., & Cho, C. (2015) Marine materials. Gene delivery, 1217–1227.
Bo, M., Bavestrello, G., Kurek, D., Paasch, S., Brunner, E., Born, R., et al. (2012). Isolation and identification of chitin in the black coral Parantipathes Larix (Anthozoa: Cnidaria). International Journal of Biological Macromolecules, 51, 129–137.
Bouhenna, M., Salah, R., Bakour, R., Drouiche, N., Abdi, N., & Mameri, N. (2015). Effects of chitin and its derivatives on human cancer cells lines. Environmental Science and Pollution Research International, 22, 15579–15586.
Brar, K., Dhillon, G., & Soccol, R. (2013). Biotransformation of waste biomass into high value biochemicals. Springer, 504, 1–29.
Chien, R.-C., Yen, M.T., & Mau, J.-L. (2015) Antimicrobial and antitumor activities of chitosan from Shiitake stipes, compared to commercial chitosan from crab shells. Carbohydrate Polymers, 1–27.
Das, S. N., Madhuprakash, J., Sarma, P. V., Purushotham, P., Suma, K., Moerschbacher, B. M., & Podile, A. (2015). Biotechnological approaches for field applications of chitooligosaccharides (COS) to induce innate immunity in plants. Critical Review in Biotechnology, 35, 29–43.
Dash, M., Chiellini, F., Ottenbrite, R. M., & Chiellini, E. (2011). Chitosan—a versatile semi-synthetic polymer in biomedical applications. Progress in Polymer Science, 36, 981–1014.
de Oliveira, C. E., Magnani, M., de Sales, C. V., Stamford, T. C., & de Souza, E. L. (2014). Effects of chitosan from Cunninghamella Elegans on virulence of post-harvest pathogenic fungi in table grapes (Vitis labrusca L.). International Journal of Food Microbiology, 171, 54–61.
Dhillon, G. S., Kaur, S., Brar, S. K., & Verma, M. (2013). Green synthesis approach: extraction of chitosan from fungus mycelia. Critical Review in Biotechnology, 33, 379–403.
Dinesh, J. V., Balkhande, P. U., Ratnakar, A. N., & Bhowate, C. (2014). Extraction and FTIR analysis of chitosan from American cockroach, Periplaneta americana. International Journal of Engineering Science and Innovative Technology, 3, 299–304.
Dos Santos, E. R., da Silva, M. C., de Souza, P. M., Nascimento, A. E., Okada, K., & Campos-Takaki, G. M. (2013). Enhancement of Cunninghamella elegans UCP/WFCC 0542 biomass and chitosan with amino acid supply. Molecules, 18, 10095–10107.
Dossey, A. T. (2010). Insects and their chemical weaponry: new potential for drug discovery. Natural Product Reports, 27, 1737–1757.
EFSA, P. (2011). Scientific opinion on the substantiation of health claims related to chitosan and reduction in body weight (ID 679, 1499), maintenance of normal blood LDL-cholesterol concentrations (ID 4663), reduction of intestinal transit time (ID 4664) and reduction of inflammation (ID 1985) pursuant to article 13(1) of regulation (EC) no 1924/2006. EFSA Journal, 9, 1–21.
Ehrlich, H., Kaluzhnaya, O. V., Brunner, E., Tsurkan, M. V., Ereskovsky, A., Ilan, M., & Worheide, G. (2013). Identification and first insights into the structure and biosynthesis of chitin from the freshwater sponge Spongilla lacustris. Journal of Structural Biology, 183, 474–483.
Fai, A. E., Stamford, T. C., Stamford-Arnaud, T. M., Santa-Cruz, P. D., da Silva, M. C., & Stamford, T. L. (2011). Physico-chemical characteristics and functional properties of chitin and chitosan produced by Mucor circinelloides using yam bean as substrate. Molecules, 16, 7143–7154.
Fatemeh, M. Y., Hojjat, T., Sohrab, N. (2015) Chitin from Penaeus merguiensis via microbial fermentationprocessing and antioxidant activity. International Journal of Biological Macromolecules, 1–5.
Flores-Albino, B., Castillo, A., Arias, L., Gimeno, M., Go, J., & Shirai, K. (2012). Chitin and L(+)-lactic acid production from crab (Callinectes bellicosus) wastes by fermentation of Lactobacillus sp. B2 using sugar cane molasses as carbon source. Bioprocess and Biosystems Engineering, 35, 1193–1200.
Francisco, F., Simora, M., & Nuñal, S. (2015). Deproteination and demineralization of shrimp waste using lactic acid bacteria for the production of crude chitin and chitosan. AACL Bioflux, 8.
Garg, T., Rath, G., & Goyal, A. K. (2015). Biomaterials-based nanofiber scaffold: targeted and controlled carrier for cell and drug delivery. Journal of Drug Target, 23, 202–221.
Gastebois, A., Clavaud C., Aimanianda, V., & Latgé, J. P. (2009). Aspergillus fumigatus: cell wall polysaccharides, their biosynthesis and organization. Future Microbiology, 4(5), 583–595.
Ghorbel-Bellaaj, O., Younes, I., Maalej, H., Hajji, S., & Nasri, M. (2012). Chitin extraction from shrimp shell waste using Bacillus bacteria. International Journal of Biological Macromolecules, 51, 1196–1201.
Ghorbel, B., Hajjia, S., Younesa, I., Chaabounib, M., Nasria, M., & Jelloulia, M. (2013). Optimization of chitin extraction from shrimp waste with Bacillus pumilus A1 using response surface methodology. International Journal of Biological Macromolecules, 61, 243–250.
Gortari, M. C., & Hours, R. A. (2013). Biotechnological processes for chitin recovery out of crustacean waste: a mini-review. Electronic Journal of Biotechnology, 16, 1–18.
Haddar, A., Hmidet, N., Ghorbel-Bellaaj, O., Fakhfakh-Zouari, N., Sellami-Kamoun, A., & Nasri, M. (2011). Alkaline proteases produced by Bacillus licheniformis RP1 grown on shrimp wastes: application in chitin extraction, chicken feather-degradation and as a dehairing agent. Biotechnology and Bioprocess Engineering, 16, 669–678.
Hadrami, E., Adam, L. R., El Hadrami, I., & Daayf, F. (2010). Chitosan in plant protection. Marine Drugs, 8, 968–987.
Hamer, S. N., Cord-Landwehr, S., Biarnes, X., Planas, A., Waegeman, H., Moerschbacher, B. M., & Kolkenbrock, S. (2015). Enzymatic production of defined chitosan oligomers with a specific pattern of acetylation using a combination of chitin oligosaccharide deacetylases. Science Report, 5, 1–8.
Harkin, C., Bruck, W. M., & Lynch, C. (2015). Isolation & identification of bacteria for the treatment of brown crab (Cancer pagurus) waste to produce chitinous material. Journal of Applied Microbiology, 118, 954–965.
Hayes, M. (2012) Chitin, chitosan and their derivatives from marine rest raw materials. Potential Food and Pharmaceutical Applications. 115–128.
Hélène, L. and Lauzon, C. (2014) The value of chitosan. 23–35.
Hemantaranjan, A. (2014). A future perspective in crop protection: chitosan and its oligosaccharides. Advances in Plants & Agriculture Research, 1, 1–8.
Hoffmann, K., Daum, G., Koster, M., Kulicke, W., Meyer-Rammes, H., Bisping, B., & Meinhardt, F. (2010). Genetic improvement of Bacillus licheniformis strains for efficient deproteinization of shrimp shells and production of high-molecular-mass chitin and chitosan. Applied and Environmental Microbiology, 76, 8211–8221.
Hongcai, Z., Shoufeng, Y., Jiyang, F., Yun, D., Danfeng, W., & Yanyun, Z. (2014). Optimization of the fermentation conditions of Rhizopus japonicus M193 for the production of chitin deacetylase and chitosan. Carbohydrate Polymers, 101, 57–67.
Hung, W., Fang, C., Su, C., Lai, W., Chang, Y., & Tsai, T. (2000) Cytotoxicity and immunogenicity of Sacchachitin and its mechanism of action on skin wound healing. 94–100.
Jayakumar, R., Menon, D., Manzoor, K., Nair, S. V., & Tamura, H. (2010). Biomedical applications of chitin and chitosan based nanomaterials-a short review. Carbohydrate Polymers, 82, 227–232.
Kadouche, S., Lounici, H., Benaoumeur, K., Drouiche, N., Hadioui, M., & Sharrock, P. (2012). Enhancement of sedimentation velocity of heavy metals loaded hydroxyapatite using chitosan extracted from shrimp waste. Journal of Polymers and the Environment, 20, 848–857.
Kardas, I., Struszczyk, M., Kucharska, M., Lambertus, A. M., Broek, V., Jan, E. G., & Ciecha, D. (2013) Chitin and chitosan as functional biopolymers for industrial applications. Springer, 329–373.
Karimi, K., & Zamani, A. (2013). Mucor indicus: biology and industrial application perspectives: a review. Biotechnology Advances, 31, 466–481.
Kaur, K., Dattajirao, V., Shrivastava, V., & Bhardwaj, U. (2012). Isolation and characterization of chitosan-producing bacteria from beaches of Chennai, India. Enzyme Research, 2012, 1–7.
Kaur, S., & Dhillon, G. S. (2015). Recent trends in biological extraction of chitin from marine shell wastes: a review. Critical Review in Biotechnology, 35, 44–61.
Kaya, M., Akata, I., Baran, T., & Menteş, A. (2014). Physicochemical properties of chitin and chitosan produced from medicinal fungus (Fomitopsis pinicola). Food Biophysics, 10, 162–168.
Kaya, M., Akyuz, B., Bulut, E., Sargin, I., Tan, G., Erdonmez, D., et al. (2016). DNA interaction, antitumor and antimicrobial activities of three-dimensional chitosan ring produced from the body segments of a diplopod. Carbohydrate Polymer, 146, 80–89.
Kaya, M., Baran, T., Asan-Ozusaglam, M., Cakmak, Y. S., Tozak, K. O., Mol, A., et al. (2015). Extraction and characterization of chitin and chitosan with antimicrobial and antioxidant activities from cosmopolitan Orthoptera species (Insecta). Biotechnology and Bioprocess Engineering, 20, 168–179.
Kaya, M., Baran, T., Erdogan, S., Mentes, A., Ozusaglam, M. A., & Cakmak, Y. S. (2014). Physicochemical comparison of chitin and chitosan obtained from larvae and adult Colorado potato beetle (Leptinotarsa decemlineata). Materials Science & Engineering, 45, 72–81.
Kaya, M., Baran, T., & Karaarslan, M. (2015). A new method for fast chitin extraction from shells of crab, crayfish and shrimp. Natural Product Research, 29, 1477–1480.
Kaya, M., Baran, T., Mentes, A., Asaroglu, M., Sezen, G., & Tozak, K. O. (2014). Extraction and characterization of α-chitin and chitosan from six different aquatic invertebrates. Food Biophysics, 9, 145–157.
Kaya, M., Baublys, V., Can, E., Šatkauskienė, I., Bitim, B., Tubelytė, V., & Baran, T. (2014). Comparison of physicochemical properties of chitins isolated from an insect (Melolontha melolontha) and a crustacean species (Oniscus asellus). Zoomorphology, 133, 285–293.
Kaya, M., Baublys, V., Satkauskiene, I., Akyuz, B., Bulut, E., & Tubelyte, V. (2015). First chitin extraction from Plumatella repens (Bryozoa) with comparison to chitins of insect and fungal origin. International Journal of Biological Macromolecules, 79, 126–132.
Kaya, M., Erdogan, S., Mol, A., & Baran, T. (2015). Comparison of chitin structures isolated from seven Orthoptera species. International Journal of Biological Macromolecules, 72, 797–805.
Kaya, M., Lelesius, E., Nagrockaite, R., Sargin, I., Arslan, G., Mol, A., et al. (2015). Differentiations of chitin content and surface morphologies of chitins extracted from male and female Grasshopper species. PloS One, 10, 1–14.
Kaya, M., Seyyar, O., Baran, T., & Turkes, T. (2014). Bat guano as new and attractive chitin and chitosan source. Front Zoology, 1–10.
Khorrami, G. D., Younesi, Y., & Amini, G. (2011). Growth kinetics and demineralization of shrimp shell using Lactobacillus plantarum PTCC 1058 on various carbon sources Iranica. Journal of Energy and Environment, 4, 320–325.
Kim, S. K. (2011) Chitin, Chitosan, Oligosaccharides and their derivatives—biological activities and applications. CRC Press, 3–69
Kim, Y., & Park, R. (2015). Progress in bioextraction processes of chitin from crustacean biowastes. Journal of the Korean Society for Applied Biological Chemistry, 58, 545–554.
Kreyenschulte, D., Krull, R., & Margaritis, A. (2014). Recent advances in microbial biopolymer production and purification. Critical Review in Biotechnol, 34, 1–15.
Krishnaveni, B., & Ragunathan, R. (2015). Extraction and characterization of chitin and chitosan from Aspergillus terreus sps, synthesis of their bionanocomposites and study of their productive applications. Journal of Chemical and Pharmaceutical Research, 2, 115–132.
Kytosan. (2013) Chitosan Global market., 1–12, www. drug-dev.com/Main/Current-News/Kytosan-USA.
Latha, S., Suresh, G., & Ramesh, B. (2013). Studies on chitosan production from different fungal mycelium. International Journal of Current Biotechnology, 1, 9–11.
Li-Chun, C., Jian-Wen, W., Szu-Han, W., Mei-Hui, C., & Tsai, C.-H. (2014). Production and isolation of chitosan from Aspergillus terreus. Journal of Applied Polymer Science, 131, 1–8.
Liu, P., Liu, S., Guo, N., Mao, X., Lin, H., Xue, C., & Wei, D. (2014). Cofermentation of Bacillus licheniformis and Gluconobacter oxydans for chitin extraction from shrimp waste. Biochemical Engineering Journal, 91, 10–15.
Liu, S., Sun, J., Yu, L., Zhang, C., Bi, J., Zhu, F., et al. (2012). Extraction and characterization of chitin from the beetle Holotrichia parallela Motschulsky. Molecules, 17, 4604–4611.
Liu, X., Zhi, X., Liu, Y., Wu, B., Sun, Z., & Shen, J. (2012). Effect of chitosan, O-carboxymethyl chitosan, and N-[(2-hydroxy-3-N,N-dimethylhexadecyl ammonium)propyl] chitosan chloride on overweight and insulin resistance in a murine diet-induced obesity. Journal of Agriculture Food Chemical, 60, 3471–3476.
Logesh, A. R., Thillaimaharani, K. A., Sharmila, K., Kalaiselvam, M., & Raffi, S. M. (2012). Production of chitosan from endolichenic fungi isolated from mangrove environment and its antagonistic activity. Asian Pacific Journal of Tropical Biomedicine, 2, 140–143.
Marei, N. H., El-Samie, E. A., Salah, T., Saad, G. R., & Elwahy, A. H. (2015) Isolation and characterization of chitosan from different local insects in Egypt. International Journal of Biological Macromolecules, 1–7.
Mati-Baouche, N., Elchinger, P.-H., De Baynast, H., Pierre, G., Delattre, C., & Michaud, P. (2014). Chitosan as an adhesive. European Polymer Journal, 60, 198–212.
Mesa Ospina, N., Ospina Alvarez, S. P., Escobar Sierra, D. M., & Ossa Orozco, C. P. (2015). Isolation of chitosan from Ganoderma lucidum mushroom for biomedical applications. Journal of Materials Science. Materials in Medicine, 26, 135.
Mhurchu, C. N., Poppitt, S. D., McGill, A. T., Leahy, F. E., Bennett, D. A., Lin, R. B., & Rodgers, A. (2004). The effect of the dietary supplement, chitosan, on body weight: a randomised controlled trial in 250 overweight and obese adults. International journal of obesity and related metabolic disorders. Journal of the International Association for the Study of Obesity, 28, 1149–1156.
Gharieb, M. M., El-Sabbagh, S. M., & M. A. S. a. O. M. D. (2015). Production of chitosan from different species of zygomycetes and its antimicrobial activity. International Journal of Scientific & Engineering Research, 6, 123–130.
Morganti, P. (2013). Saving the environment by nanotechnology and waste raw materials: use of chitin nanofibrils by EU research projects. Applied Cosmetol, 31, 89–96.
Motawie, A. M., Mahmoud, K. F., El-Sawy, A. A., Kamal, H. M., Hefni, H., & Ibrahiem, H. A. (2014). Preparation of chitosan from the shrimp shells and its application for pre-concentration of uranium after cross-linking with epichlorohydrin. Egyptian Journal of Petroleum, 23, 221–228.
Navard, P. (2012). The European polysaccharide. Network (EPNOE)., 1–393.
Nitschke, J., Altenbach, H. J., Malolepszy, T., & Molleken, H. (2011). A new method for the quantification of chitin and chitosan in edible mushrooms. Carbohydrate Research, 346, 1307–1310.
Nouri, M., Khodaiyan, F., Razavi, S., & Mousavi, M. (2015). Improvement of chitosan production from Persian gulf shrimp waste by response surface methodology. Food Hydrocolloids, 1–9.
Nwe, N., & Furuit, T. (2011). Chitosan from aquatic and terrestrial organisms and microorganisms. In S.-K. Kim (Ed.), Chitin, chitosan, oligosaccharides and their derivatives (pp. 3–10). New York: CRC Press.
Nwe, N., & Stevens, W. F. (2004). Effect of urea on fungal chitosan production in solid substrate fermentation. Process Biochemistry, 39, 1639–1642.
Ospina Alvarez, S. P., Ramirez Cadavid, D. A., Escobar Sierra, D. M., Ossa Orozco, C. P., & Rojas Vahos, D. F. (2014). Comparison of extraction methods of chitin from Ganoderma lucidum mushroom obtained in submerged culture. BioMedical Research International, 2014, 1–7.
Pacheco, N., Garnica-Gonzalez, M., Gimeno, M., Barzana, E., Trombotto, S., & David, L. S. K. (2011). Structural characterization of chitin and chitosan obtained by biological and chemical methods. Biomacromolecules, 12, 3285–3290.
Pareek, N., Vivekanand, V., Agarwal, P., Saroj, S., & Singh, R. P. (2013). Bioconversion to chitosan: a two stage process employing chitin deacetylase from Penicillium oxalicum SAEM-51. Carbohydrate Polymer, 96, 417–425.
Pareek, N., Vivekanand, V., Singh, R., & Pletschke, P. (2013). Advances in enzyme biotechnology, chitin deacetylase: characteristic molecular features and functional aspects. Springer, 125–136.
Park, B. K., & Kim, M. M. (2010). Applications of chitin and its derivatives in biological medicine. International Journal of Molecular Sciences, 11, 5152–5164.
Paul, T., Halder, S., Das, A., Ghosh, K., Mandal, A., Payra, P., et al. (2014). Production of chitin and bioactive materials from black tiger shrimp (Penaeus monodon) shell waste by the treatment of bacterial protease cocktail. 3. Biotechnology, 5, 483–493.
Pires, C. T., Vilela, J. A., & Airoldi, C. (2014). The effect of chitin alkaline deacetylation at different condition on particle properties. Procedia Chemistry, 9, 220–225.
Ploydee, E. and Chaiyanan, S. (2014) Production of high viscosity chitosan from biologically purified chitin isolated by microbial fermentation and deproteinization. International Journal of Polymer Science, 1–8.
Prameela, K., Mohan, C., Smitha, P., & Hemalatha, K. (2010). Bioremediation of shrimp biowaste by using natural probiotic for chitin and carotenoid production an alternative method to hazardous chemical method. International Journal of Applied Biology and Pharmaceutical Technology, 1, 903–910.
Priya, D., Suriyaprabha, R., Yuvakkumar, R., & Rajendran, V. (2014). Chitosan-incorporated different nanocomposite HPMC films for food preservation. Journal of Nanoparticle Research, 16, 1–12.
Puvvada, Y., Vankayalapati, S., & Sukhavasi, S. (2012). Extraction of chitin from chitosan from exoskeleton of shrimp for application in the pharmaceutical industry. International Current Pharmaceutical Journal, 1, 258–263.
Radwan, M. A., Farrag, S. A., Abu-Elamayem, M. M., & Ahmed, N. S. (2011). Extraction, characterization, and nematicidal activity of chitin and chitosan derived from shrimp shell wastes. Biology and Fertility of Soils, 48, 463–468.
Ragunathan, R., & Krishnaveni, K. (2015). Extraction and characterization of chitin and chitosan from Aspergillus terreus sps, synthesis of their bionanocomposites and study of their productive applications. Journal of Chemical Pharmaceutical Research, 2, 115–132.
Rao, M. S., & Stevens, W. F. (2005). Chitin production by Lactobacillus fermentation of shrimp biowaste in a drum reactor and its chemical conversion to chitosan. Journal of Chemical Technology & Biotechnology, 80, 1080–1087.
Sachindra, N., Bhaskar, N., & Mahendrakar, N. (2005). Carotenoids in different body components of Indian shrimps. Journal of the Science of Food and Agriculture, 85, 167–172.
Sachindra, N., & Mahendrakar, N. (2010). Stability of carotenoids recovered from shrimp waste and their use as colorant in fi sh sausage. Journal of Food Science Technology, 47, 77–83.
Safaei, Z., Karimi, K., Golkar, P., & Zamani, A. (2015). Effects of plant growth hormones on Mucor Indicus growth and chitosan and ethanol production. International Journal of Molecular Sciences, 16, 16683–16694.
Salah, R., Michaud, P., Mati, F., Harrat, Z., Lounici, H., Abdi, N., & Drouiche, N. (2013). Anticancer activity of chemically prepared shrimp low molecular weight chitin evaluation with the human monocyte leukaemia cell line, THP-1. International Journal of Biological Macromolecules, 52, 333–339.
Santas, J., Espadaler, J., Mancebo, R., & Rafecas, M. (2012). Selective in vivo effect of chitosan on fatty acid, neutral sterol and bile acid excretion: a longitudinal study. Food Chemistry, 134, 940–947.
Sathiyabama, M., & Charles, R. E. (2015). Fungal cell wall polymer based nanoparticles in protection of tomato plants from wilt disease caused by Fusarium oxysporum f.Sp. lycopersici. Carbohydrate Polymer, 133, 400–407.
Sayari, N., Sila, A., Abdelmalek, B. E., Abdallah, R. B., Ellouz-Chaabouni, S., Bougatef, A., & Balti, R. (2016). Chitin and chitosan from the Norway lobster by-products: antimicrobial and anti-proliferative activities. International Journal of Biological Macromolecules, 87, 163–171.
Singh, V., Malviya, T., & Sanghi, R. (2012) Polysaccharide-based macromolecular materials for decolorization of textile effluent. Springer, 377–403.
Sinha, S., Chand, S., & Tripathi, P. (2016) Recent progress in chitosanase production of monomer-Free chitooligosaccharides: Bioprocess strategies and Future Applications. Applied Biochemistry and Biotechnology.
Tao, W., Zivanovic, S., Draughon, F., Conway, W., & Sams, C. (2005). Physicochemical properties and bioactivity of fungal chitin and chitosan. Journal of Agriculture Food Chemistry., 52, 3888–3894.
Vaingankar, P. N., & Juvekar, A. R. (2014). Fermentative production of mycelial chitosan from zygomycetes: media optimization and physico-chemical characterization. Advances in Bioscience and Biotechnology, 05, 940–956.
Vasiliev, Y. (2015). Chitosan-based vaccine adjuvants incomplete characterization complicates preclinical and clinical evaluation. Expert Research Vaccines, 14, 37–53.
Venkat, R. F., & Yatsenko, L. (2012) Nano-bio architectures: combining chemistry and biology in nanotechnology. Springer, 3–24.
Venkatesan, J. and Kim, S. (2013) Marine Biomaterials. 3–23.
Vilela, P., Joice, A. P., & Airoldi, C. (2014). The effect of chitin alkaline deacetylation at different condition on particle properties. Procedia Chemistry, 9, 220–225.
Vo, T., & Kim, S.-k. (2014) Chitin and Its Beneficial Activity as an Immunomodulator in allergic reactions. Springer 361–369.
Wassila, A., Adour, L., & Amrane, A. (2013). Chitin extraction from crustacean shells using biological methods. Food Technology and Biotechnology, 51, 12–25.
Wysokowski, M., Bazhenov, V. V., Tsurka, M. V., Galli, R., Stelling, A. L., Stocker, H., & Kaiser, S. (2013). Isolation and identification of chitin in three-dimensional skeleton of Aplysina fistularis marine sponge. International Journal of Biological Macromolecules, 62, 94–100.
Younes, I., Ghorbel-Bellaaj, O., Nasri, R., Chaabouni, M., Rinaudo, M., & Nasri, M. (2012). Chitin and chitosan preparation from shrimp shells using optimized enzymatic deproteinization. Process Biochemistry, 47, 2032–2039.
Younes, I., Hajji, S., Frachet, V., Rinaudo, M., Jellouli, K., & Nasri, M. (2014). Chitin extraction from shrimp shell using enzymatic treatment. Antitumor, antioxidant and antimicrobial activities of chitosan. International Journal of Biological Macromolecules, 69, 489–498.
Yuan, Y., Sun, Y. E., Wan, Z. L., Yang, X. Q., Wu, J. F., & Yin, S. W. (2014). Chitin microfibers reinforce soy protein gels cross-linked by transglutaminase. Journal of Agriculture Food Chemistry, 62, 4434–4442.
Zhang, D., Jin, Y., Deng, Y., Wang, D., & Zhao, Y. (2012). Production of chitin from shrimp shell powders using Serratia Marcescens B742 and Lactobacillus plantarum ATCC 8014 successive two-step fermentation. Carbohydrate Research, 362, 13–20.
Zhao, Y., Park, R. D., & Muzzarelli, R. A. (2010). Chitin deacetylases: properties and applications. Marine Drugs, 8, 24–46.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this manuscript.
Ethical Statement
The article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Philibert, T., Lee, B.H. & Fabien, N. Current Status and New Perspectives on Chitin and Chitosan as Functional Biopolymers. Appl Biochem Biotechnol 181, 1314–1337 (2017). https://doi.org/10.1007/s12010-016-2286-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2286-2