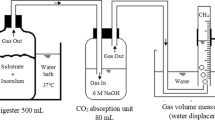
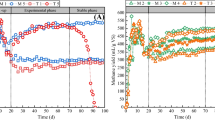
Abstract
The effects of hydraulic retention time (HRT), pH, and operating temperature (T OP) on the degradation of food waste-recycling wastewater (FRW) were investigated in laboratory-scale hydrolysis/acidogenesis reactors. Response surface analysis was used to approximate the production of volatile organic acids and degradation of volatile suspended solids (VSS), carbohydrate, protein, and lipid with regard to the independent variables (1 ≤ HRT ≤ 3 days, 4 ≤ pH ≤ 6, 25 ≤ T OP ≤ 45 °C). Partial cubic models adequately approximated the corresponding response surfaces at α < 5 %. The physiological conditions for maximum acidification (0.4 g TVFA + EtOH/g VSadded) and the maximal degradation of VSS (47.5 %), carbohydrate (92.0 %), protein (17.7 %), and lipid (73.7 %) were different. Analysis of variance suggested that pH had a great effect on the responses in most cases, while T OP and HRT, and their interaction, were significant in some cases. Denaturing gradient gel electrophoresis analysis revealed that Sporanaerobacter acetigenes, Lactobacillus sp., and Eubacterium pyruvivorans-like microorganisms might be main contributors to the hydrolysis and acidogenesis of FRW. Biochemical methane potential test confirmed higher methane yield (538.2 mL CH4/g VSadded) from an acidogenic effluent than from raw FRW.






Similar content being viewed by others
References
Angelidaki, I., & Sanders, W. (2004). Assessment of the anaerobic biodegradability of macropollutants. Reviews in Environmental Science and Biotechnology, 3, 117–129.
APHA–AWWA–WEF. (2005). Standard methods for the examination of water and wastewater (21st ed.). Washington, DC: American Public Health Association.
Atkinson, B., & Mavituna, F. (1991). Biochemical engineering and biotechnology handbook (2nd ed.). New York: Nature Press.
Batstone, D. J., Keller, J., Angelidaki, I., Kalyuzhnyi, S. V., Pavlostathis, S. G., Rozzi, A., Sanders, W. T., Siegrist, H., & Vavilin, V. A. (2002). The IWA Anaerobic Digestion Model No 1 (ADM1). Water Science and Technology: A Journal of the International Association on Water Pollution Research, 45, 65–73.
Bligh, E. G., & Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Canadian Journal of Biochemistry and Physiology, 37, 911–917.
Bodkhe, S. (2008). Development of an improved anaerobic filter for municipal wastewater treatment. Bioresource Technology, 99, 222–226.
Cammarota, M. C., & Freire, D. M. G. (2006). A review on hydrolytic enzymes in the treatment of wastewater with high oil and grease content. Bioresource Technology, 97, 2195–2210.
Choi, Y. J., Hur, S., Choi, B.-D., Konno, K., & Park, J. W. (2009). Enzymatic hydrolysis of recovered protein from frozen small croaker and functional properties of its hydrolysates. Journal of Food Science, 74, C17–C24.
Cirne, D. G., Delgado, O. D., Marichamy, S., & Mattiasson, B. (2006). Clostridium lundense sp. nov., a novel anaerobic lipolytic bacterium isolated from bovine rumen. International Journal of Systematic and Evolutionary Microbiology, 56, 625–628.
Dighe, A. S., Shouche, Y. S., & Ranade, D. R. (1998). Selenomonas lipolytica sp. nov., an obligately anaerobic bacterium possessing lipolytic activity. International Journal of Systematic Bacteriology, 48, 783–791.
DuBois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28, 350–356.
Elefsiniotis, P., & Oldham, W. (1994). Substrate degradation patterns in acid-phase anaerobic digestion of municipal primary sludge. Environmental Technology, 15, 741–751.
Follmann, M., Ochrombel, I., Kramer, R., Trotschel, C., Poetsch, A., Ruckert, C., Huser, A., Persicke, M., Seiferling, D., Kalinowski, J., & Marin, K. (2009). Functional genomics of pH homeostasis in Corynebacterium glutamicum revealed novel links between pH response, oxidative stress, iron homeostasis and methionine synthesis. BMC Genomics, 10, 621.
Foresti, E., Zaiat, M., & Vallero, M. (2006). Anaerobic processes as the core technology for sustainable domestic wastewater treatment: consolidated applications, new trends, perspectives, and challenges. Reviews in Environmental Science and Bio/Technology, 5, 3–19.
Ganesh, R., Torrijos, M., Sousbie, P., Lugardon, A., Steyer, J. P. and Delgenes, J. P. (2014). Single-phase and two-phase anaerobic digestion of fruit and vegetable waste: comparison of start-up, reactor stability and process performance. Waste Management, 34, 875–885.
Garrity, G., Brenner, D. J., Krieg, N. R. and Staley, J. R. (2005). Bergey’s manual® of systematic bacteriology, 2nd ed. New York: Springer.
Green, S. J., Leigh, M. B. and Neufeld, J. D. (2010). Denaturing gradient gel electrophoresis (DGGE) for microbial community analysis. In K. N. Timmis (Ed.), Handbook of hydrocarbon and lipid microbiology (pp. 4137–4158). Heidelberg: Springer.
Guerrero, L., Omil, F., Méndez, R., & Lema, J. M. (1999). Anaerobic hydrolysis and acidogenesis of wastewaters from food industries with high content of organic solids and protein. Water Research, 33, 3281–3290.
Hernandez-Eugenio, G., Fardeau, M.-L., Cayol, J.-L., Patel, B. K. C., Thomas, P., Macarie, H., Garcia, J.-L., & Ollivier, B. (2002). Sporanaerobacter acetigenes gen. nov., sp. nov., a novel acetogenic, facultatively sulfur-reducing bacterium. International Journal of Systematic and Evolutionary Microbiology, 52, 1217–1223.
Hung, C.-H., Chang, Y.-T., & Chang, Y.-J. (2011). Roles of microorganisms other than Clostridium and Enterobacter in anaerobic fermentative biohydrogen production systems—a review. Bioresource Technology, 102, 8437–8444.
Jiang, J., Zhang, Y., Li, K., Wang, Q., Gong, C., & Li, M. (2013). Volatile fatty acids production from food waste: effects of pH, temperature, and organic loading rate. Bioresource Technology, 143, 525–530.
Jo, J. H., Jeon, C. O., Lee, D. S., & Park, J. M. (2007). Process stability and microbial community structure in anaerobic hydrogen-producing microflora from food waste containing kimchi. Journal of Biotechnology, 131, 300–308.
Kim, M., & Chun, J. (2005). Bacterial community structure in kimchi, a Korean fermented vegetable food, as revealed by 16S rRNA gene analysis. International Journal of Food Microbiology, 103, 91–96.
Kim, M., Gomec, C. Y., Ahn, Y., & Speece, R. E. (2003). Hydrolysis and acidogenesis of particulate organic material in mesophilic and thermophilic anaerobic digestion. Environmental Technology, 24, 1183–1190.
Kim, M., Song, M., Jo, M., Shin, S., Khim, J., & Hwang, S. (2010). Growth condition and bacterial community for maximum hydrolysis of suspended organic materials in anaerobic digestion of food waste-recycling wastewater. Applied Microbiology and Biotechnology, 85, 1611–1618.
Kim, W., Ryu, B.-G., Kim, S., Heo, S.-W., Kim, D., Kim, J., Jo, H., Kwon, J.-H., & Yang, J.-W. (2014). Quantitative analysis of microbial community structure in two-phase anaerobic digesters treating food wastewater. Korean Journal of Chemical Engineering, 31, 381–385.
Kim, Y.-O., Kim, K.-K., Park, S., Kang, S.-J., Lee, J.-H., Lee, S.-J., Oh, T.-K., & Yoon, J.-H. (2010). Photobacterium gaetbulicola sp. nov., a lipolytic bacterium isolated from a tidal flat sediment. International Journal of Systematic and Evolutionary Microbiology, 60, 2587–2591.
Lee, J., Hwang, B., Koo, T., Shin, S. G., Kim, W., & Hwang, S. (2014). Temporal variation in methanogen communities of four different full-scale anaerobic digesters treating food waste-recycling wastewater. Bioresource Technology, 168, 59–63.
Lim, B. S., Kim, B., & Chung, I. (2012). Anaerobic treatment of food waste leachate for biogas production using a novel digestion system. Environmental Engineering Research, 17, 41–46.
Lim, S. J., Kim, B. J., Jeong, C. M., Choi, J. D. R., Ahn, Y. H., & Chang, H. N. (2008). Anaerobic organic acid production of food waste in once-a-day feeding and drawing-off bioreactor. Bioresource Technology, 99, 7866–7874.
Lu, J., Gavala, H. N., Skiadas, I. V., Mladenovska, Z., & Ahring, B. K. (2008). Improving anaerobic sewage sludge digestion by implementation of a hyper-thermophilic prehydrolysis step. Journal of Environmental Management, 88, 881–889.
Mahmoud, N., Zeeman, G., Gijzen, H., & Lettinga, G. (2004). Anaerobic stabilisation and conversion of biopolymers in primary sludge—effect of temperature and sludge retention time. Water Research, 38, 983–991.
McInerney, M. J. (1988). Anaerobic hydrolysis and fermentation of fats and proteins. In A. J. B. Zehnder (Ed.), Biology of anaerobic microorganisms (pp. 373–416) New York: Wiley.
Min, K. S., Khan, A. R., Kwon, M. K., Jung, Y. J., Yun, Z., & Kiso, Y. (2005). Acidogenic fermentation of blended food-waste in combination with primary sludge for the production of volatile fatty acids. Journal of Chemical Technology & Biotechnology, 80, 909–915.
Min, K. S., Park, K. S., Jung, Y. J., Khan, A. R., & Kim, Y. J. (2002). Acidogenic fermentation: utilization of wasted sludge as a carbon source in the denitrification process. Environmental Technology, 23, 293–302.
MOE. (2013). National waste statistical survey, 제4차 전국폐기물통계조사. Republic of Korea: Ministry of Environment.
Muyzer, G., De Waal, E., & Uitterlinden, A. (1993). Profiling of complex microbial population by DGGE analysis of polymerase chain reaction amplified genes coding for 16S rRNA. Applied and Environmental Microbiology, 59, 695–700.
Myerson, A. (Ed.) (2002). Handbook of industrial crystallization. Newton, MA: Butterworth-Heinemann.
Ofori-Boateng, C., & Lee, K. T. (2014). Ultrasonic-assisted simultaneous saccharification and fermentation of pretreated oil palm fronds for sustainable bioethanol production. Fuel, 119, 285–291.
Puolanne, E., & Halonen, M. (2010). Theoretical aspects of water-holding in meat. Meat Science, 86, 151–165.
Ramsay, I. R., & Pullammanappallil, P. C. (2001). Protein degradation during anaerobic wastewater treatment: derivation of stoichiometry. Biodegradation, 12, 247–256.
Romanenko, L., Lysenko, A., Rohde, M., Mikhailov, V., & Stackebrandt, E. (2004). Psychrobacter maritimus sp. nov. and Psychrobacter arenosus sp. nov., isolated from coastal sea ice and sediments of the Sea of Japan. International Journal of Systematic and Evolutionary Microbiology, 54, 1741.
Romero Aguilar, M. A., Fdez-Güelfo, L. A., Álvarez-Gallego, C. J., & Romero García, L. I. (2013). Effect of HRT on hydrogen production and organic matter solubilization in acidogenic anaerobic digestion of OFMSW. Chemical Engineering Journal, 219, 443–449.
Ruiz, E., Cara, C., Ballesteros, M., Manzanares, P., Ballesteros, I., & Castro, E. (2006) Ethanol production from pretreated olive tree wood and sunflower stalks by an SSF process. Applied Biochemistry and Biotechnology, 130, 631–643.
Shin, S. G., Han, G., Lee, J., Cho, K., Jeon, E.-J., Lee, C., & Hwang, S. (2015). Characterization of food waste-recycling wastewater as biogas feedstock. Bioresource Technology, 196, 200–208.
Shin, S. G., Han, G., Lim, J., Lee, C., & Hwang, S. (2010). A comprehensive microbial insight into two-stage anaerobic digestion of food waste-recycling wastewater. Water Research, 44, 4838–4849.
Sikorski, Z. E. (Ed.) (2006) Chemical and functional properties of food components, third edition. Boca Raton, FL: CRC.
Speece, R. E. (1996). Anaerobic biotechnology for industrial wastewaters. Nashville, TN: Archae Press.
Vaclavik, V. and Christian, E. W. (2013). Essentials of food science. New York: Springer.
Vavilin, V. A., Fernandez, B., Palatsi, J., & Flotats, X. (2008). Hydrolysis kinetics in anaerobic degradation of particulate organic material: an overview. Waste Management, 28, 939–951.
Veeken, A., & Hamelers, B. (1999). Effect of temperature on hydrolysis rates of selected biowaste components. Bioresource Technology, 69, 249–254.
Vos, P. D. , Garrity, G. M., Jones, D., Krieg, N. R., Ludwig, W., Rainey, F. A., Schleifer, K. H. and Whitman, W. B. (Ed.) (2009). Bergey’s manual of systematic bacteriology: volume 3: The Firmicutes. New York: Springer.
Wallace, R. J., Chaudhary, L. C., Miyagawa, E., McKain, N., & Walker, N. D. (2004). Metabolic properties of Eubacterium pyruvativorans, a ruminal ‘hyper-ammonia-producing’ anaerobe with metabolic properties analogous to those of Clostridium kluyveri. Microbiology, 150, 2921–2930.
Wallace, R. J., McKain, N., McEwan, N. R., Miyagawa, E., Chaudhary, L. C., King, T. P., Walker, N. D., Apajalahti, J. H. A., & Newbold, C. J. (2003). Eubacterium pyruvativorans sp. nov., a novel non-saccharolytic anaerobe from the rumen that ferments pyruvate and amino acids, forms caproate and utilizes acetate and propionate. International Journal of Systematic and Evolutionary Microbiology, 53, 965–970.
Wang, K., Yin, J., Shen, D., & Li, N. (2014). Anaerobic digestion of food waste for volatile fatty acids (VFAs) production with different types of inoculum: effect of pH. Bioresource Technology, 161, 395–401.
Warnecke, T., & Gill, R. (2005). Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microbial Cell Factories, 4, 1–8.
Yang, K., Yu, Y., & Hwang, S. (2003). Selective optimization in thermophilic acidogenesis of cheese–whey wastewater to acetic and butyric acids: partial acidification and methanation. Water Research, 37, 2467–2477.
Yu, H. Q., & Fang, H. H. P. (2003). Acidogenesis of gelatin-rich wastewater in an upflow anaerobic reactor: influence of pH and temperature. Water Research, 37, 55–66.
Yu, Y., Lee, C., Kim, J., & Hwang, S. (2005). Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnology and Bioengineering, 89, 670–679.
Yuan, Q., Sparling, R., & Oleszkiewicz, J. A. (2011). VFA generation from waste activated sludge: effect of temperature and mixing. Chemosphere, 82, 603–607.
Zayas, J. F. (Ed.) (1996). Functionality of proteins in food. Heidelberg: Springer.
Zhang, B., He, P.-J., Fan, L., Shao, L.-M., & Wang, P. (2007). Extracellular enzyme activities during regulated hydrolysis of high-solid organic wastes. Water Research, 41, 4468–4478.
Zhang, B., Zhang, L.-L., Zhang, S.-C., Shi, H.-Z., & Cai, W.-M. (2005). The influence of pH on hydrolysis and acidogenesis of kitchen wastes in two-phase anaerobic digestion. Environmental Technology, 26, 329–339.
Acknowledgments
This work was financially supported by Korea Ministry of Environment (MOE) as “Knowledge-based environmental service (Waste to energy recycling) Human resource development Project” and also supported by “Human Resources Program in Energy Technology” of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resource from the Ministry of Trade, Industry & Energy, Republic of Korea (no. 20144030200460).
Author information
Authors and Affiliations
Corresponding author
Additional information
Gyuseong Han and Seung Gu Shin contributed equally to this work.
Rights and permissions
About this article
Cite this article
Han, G., Shin, S.G., Lee, J. et al. Mesophilic Acidogenesis of Food Waste-Recycling Wastewater: Effects of Hydraulic Retention Time, pH, and Temperature. Appl Biochem Biotechnol 180, 980–999 (2016). https://doi.org/10.1007/s12010-016-2147-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2147-z