Abstract
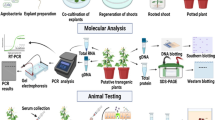
Transgenic tobacco plants were developed expressing WbSXP-1, a diagnostic antigen isolated from the cDNA library of L3 stage larvae of Wucheraria bancrofti. This antigen produced by recombinant Escherichia coli has been demonstrated by to be successful as potential diagnostic candidate against lymphatic filariasis. A rapid format simple and qualitative flow through immune-filtration diagnostic kit has been developed for the identification of IgG antibodies to the recombinant WbSXP-1 and is being marketed by M/S Span Diagnostics Ltd in India and Africa. Here, we present the results of experiments on the transformation and expression of the same filarial antigen, WbSXP-1, in tobacco plant, Nicotiana tabacum, to produce plant-based diagnostic antigen. It was possible to successfully transform the tobacco plant with WbSXP-1, the integration of the parasite-specific gene in plants was confirmed by PCR amplification and the expression of the filarial protein by Western blotting. The immunoreactivity of the plant-produced WbSXP-1 was assessed based on its reaction with the monoclonal antibodies developed against the E. coli-produced protein. Immunological screening using clinical sera from patients indicates that the plant-produced protein is comparable to E. coli-produced diagnostic antigen. The result demonstrated that plants can be used as suitable expression systems for the production of diagnostic proteins against lymphatic filariasis, a neglected tropical infectious disease which has a negative impact on socioeconomic development. This is the first report of the integration, expression and efficacy of a diagnostic candidate of lymphatic filariasis in plants.
Key Message
Transgenic tobacco plants with WbSXP-1, a filarial diagnostic candidate, were developed. The plant-produced protein showed immunoreactivity on par with the E. coli product.







Similar content being viewed by others
References
Dissanayake, S., Zheng, H., Dreyer, G., Xu, M., Watawana, L., Cheng, G., Wang, S., Morin, P., Deng, B., Kurniawan, L., Vincent, A., & Piessens, W. F. (1994). Evaluation of a recombinant parasite antigen for the diagnosis of lymphatic filariasis. American Journal of Tropical Medicine and Hygiene, 50, 727–734.
Weil, G., & Ramzy, R. M. R. (2006). Diagnostic tools for filariasis elimination programs. Trends in Parasitology, 2, 78.
Lalitha, P., Ravichandran, M., Suba, S., Kaliraj, P., Narayanan, R. B., & Jayaraman, K. (1998). Quantitative assessment of circulating antigens in human lymphatic filariasis: a field evaluation of monoclonal antibody-based ELISA using blood collected on filter strips. Tropical Medicine & International Health, 3, 41–45.
Rao, K. V. N., Eswaran, D., Ravi, V., Gnanasekhar, B., Narayanan, R. B., Kaliraj, P., Jayaraman, K., Marson, A., Nithyakalyani, R., & Scott, A. L. (2000). The Wuchereria bancrofti Orthologue of Brugia malayi SXP1 and the diagnosis of Bancroftian Filariasis. Molecular and Biochemical Parasitology, 107, 71–80.
Lalitha, P., Eswaran, D., Gnanasekar, M., Rao, K. V. N., Narayanan, R. B., Scott, A. L., Nutman, T. B., & Kaliraj, P. (2002). Development of antigen detection ELISA for the diagnosis of brugian and bancroftian filariasis using antibodies to recombinant filarial antigens BmSXP-1 and Wb-SXP-1. Microbiology and Immunology, 46(5), 327–332.
Rahmah, N., Lim, B. H., Anuar, A. K., Shenoy, R. K., Kumaraswami, V., Hakim, S. L., Chotechuang, P., Kanjanopas, K., & Ramachandran, C. P. (2001). A recombinant antigen-based IgG4 ELISA for the specific and sensitive detection of Brugia malayi infection. Transactions of the Royal Society of Tropical Medicine and Hygiene, 95, 280–284.
Rahmah, N., Taniawati, S., Shenoy, R. K., Lim, B. H., Kumaraswami, V., Anuar, A. K., Hakim, S. L., Hayati, M. I. N., Chan, B. T., Suharni, M., & Ramachandran, C. P. (2001). Specificity and sensitivity of a rapid dipstick test (Brugia Rapid) in the detection of Brugia malayi infection. Transactions of the Royal Society of Tropical Medicine and Hygiene, 95, 601–604.
More, S. J., & Copeman, D. B. (1990). A highly specific and sensitive monoclonal antibody-based ELISA for the detection of circulating antigen in bancroftian filariasis. Tropical Medicine and Parasitology, 41, 403–406.
Weil, G. J., Lammie, P. J., & Weiss, N. (1997). The ICT filariasis test: a rapid format antigen test for diagnosis of Bancroftian filariasis. Parasitology Today, 13, 401–4041. –80.
Bhaskar, V., Kanthan, L., Srikanth, T., Suba, S., Mody, H., Desai, P., & Kaliraj, P. (2004). Development and evaluation of a rapid flow through immunofiltration test using recombinant filarial antigen for diagnosis of brugian and bancroftian filariasis. Microbiology and Immunology, 48(7), 519–525.
Wingfield, P. T., Palmer, I., Liang, S. M. (2001). Folding and purification of insoluble (inclusion body) proteins from Escherichia coli. Current Protocol Protein Science, Chapter 6: Unit 6.5. doi:10.1002/0471140864.ps0605s00.
Wacker, M., Linton, D., Hitchen, P. G., et al. (2002). N-linked glycosylation in campylobacter jejuni and its functional transfer into E. coli. Science, 298(5599), 1790–1793.
Tolia, N. H., & Joshua-Tor, L. (2006). Strategies for protein co expression in Escherichia coli. Nature Methods, 3(1), 55–64.
Mason, H. S., Lam, D. M. K., & Arntzen, C. J. (1992). Expression of hepatitis B surface antigen in transgenic plants. Proceedings of the National Academy of Science (USA), 89, 11745–11749.
Tackets, C. O., Mason, H. S., Losonsky, G., Clements, J. D., Levine, M. M., & Arntzen, C. J. (1998). Immunogenicity in humans of a recombinant bacterial antigen delivered in a transgenic potato. Nature Medicine, 4(5), 607–609.
Rosales-Mendoza, S., Soria-Guerra, R. E., Moreno-Fierros, L., Han, Y., Alpuche-solis, A. G., & Korban, S. S. (2011). Transgenic carrot tap roots expressing an immunogenic F1-V fusion protein from Yersinis pestis are immunogenic in mice. Journal of Plant Physiology, 168, 174–180.
Mathangi, G., Adhiseshan, P., Chakravarthi, M., Harunipriya, P., & Kaliraj, P. (2014). Immunogenicity of Brugia malayi Abundant Larval Transcript-2, a potential filarial vaccine candidate expressed in tobacco. Plant Cell Reports, 33, 179–188.
Kathuria, S., Sriraman, R., Nath, R., Sack, M., Pal, R., Artsaenko, O., Talwar, G. P., Fischer, R., & Finnern, R. (2002). Efficacy of plant produced recombinant antibodies against HCg. Human Reproduction, 17(8), 2054–2061.
He, J., Lai, H., Brock, C., Chen, Q. (2012). A novel system for rapid and cost-effective production of detection and diagnostic reagents of west Nile virus in plants. Journal of Biomedical Biotechnology, 1–10.
Hoekema, A., Hooykaas, P. J. J., & Schilperoort, R. A. (1984). Transfer of the octopine T-DNA segment to plant cells mediated by different types of Agrobacterium tumor- or root-inducing plasmids: generality of virulence systems. Journal of Bacteriology, 158, 383–385.
Stewart, C. N., Jr., & Via, L. E. (1993). A rapid CTAB isolation technique for RAPD fingerprint and other PCR applications. Biotechniques, 14, 748–749.
Bhandari, P., & Gowrishankar, J. (1997). An Escherichia coli host strain useful for efficient overproduction of cloned gene products with NaCl as the inducer. Journal of Bacteriology, 179, 4403–4406.
Janardhan, S., Pandiaraja, P., Thirugnanam, S., Balamurali, M. N., Fernando, K., Mody, H. C., Desai, P. K., Meenakshisundaram, S., & Kaliraj, P. (2007). Production, purification and diagnostic application of filarial recombinant protein WbSXP-1 expressed in salt inducible Escherichia coli. Journal of Industrial Microbiology & Biotechnology, 34, 675–683.
Aziz, M. A., Singh, S., Anand, K. P., & Bhatnagar, R. (2002). Expression of protective antigen in transgenic plants: a step towards edible vaccine against anthrax. Biochemical and Biophysical Research Communications, 299, 345–351.
Elkholy, S. F., Ismail, R. M., Bahieldin, A., Sadik, A. S., & Madkour, M. A. (2009). Expression of hepatitis B surface antigen (HBsAg) gene in transgenic banana (Musa sp.). Arab Journal of Biotechnology, 12, 291–302.
Iannetta, P. P. M., James, E. K., McHardy, P. D., Sprent, J. I., & Minchin, F. R. (1993). An ELISA procedure for quantification of relative amount of intercellular glycoprotein in legume nodules. Annals of Botany, 71, 85–90.
Pandey, V., Madhumathi, J., Karande, A. A., & Kaliraj, P. (2011). Antigen detection assay with parasite specific monoclonal antibodies for diagnosis of lymphatic filariasis. Clinica Chimica Acta, 412, 1867–1873.
Harlow, E., & David, L. (1998). Using antibodies: a laboratory manual (2nd ed.). Cold Spring Harbour Laboratory Press: New York.
Cardineu, G.A., Curtis, III R. (1990). Oral immunization by transgenic plants. Patent, WO 1990002484 March 23, 1998.
Bouche, F. B., Marquet-Blouin, E., Yanagi, Y., Steinmetz, A., & Muller, C. P. (2003). Neutralizing immunogenicity of a polyepitope antigen expressed in a transgenic food plant: a novel antigen to protect against measles. Vaccine, 21, 2065–2072.
Sunilkumar, G. B., Ganapathi, T. R., Revathi, C. J., Prasad, K. S. N., & Bapat, V. A. (2003). Expression of hepatitis B surface antigen in tobacco cell suspension cultures. Protein Expression and Purification, 32, 10–17.
Arlen, P. A., Singleton, M., Adamovicz, J. J., Ding, Y., Davoodi-Semiromi, A., & Daniel, H. (2008). Effective plague vaccination via oral delivery of plant cells expressing F1-Vs in chloroplasts. Infection and Immunity, 76, 3640–3650.
Barta, A., Sommengruber, K., Thompson, D., Hartmuth, K., Matzke, M., & Matzke, A. (1986). The expression of nopaline synthase human growth hormone chimeric gene in transformed tobacco and sunflower callus tissue. Plant Molecular Biology, 6, 347–357.
Staub, J. M., Garcia, B., Graves, J., Hajdukiewwicz, P. T., Hunter, P., Nehra, N., Paradkar, V., Schlittler, M., Carroll, J. A., Spatola, L., Ward, D., Ye, G., & Russel, D. A. (2000). High yield production of a human therapeutic protein in tobacco chloroplasts. Nature Biotechnology, 18(3), 333–338.
Zhu, Z., Hughes, K., Huang, L., Sun, B., Liu, C., Li, Y. (1994). Expression of human α-interferon cDNA in transgenic rice plants. Plant Cell, Tissue and Organ Culture, 36(2), 197–204.
Ruggiero, F., Exposito, J.-Y., Bournat, P., Gruber, V., Perret, S., Comte, J., Olagnier, B., Garrone, R., & Theisen, M. (2000). Triple helix assembly and processing of human collagen produced in transgenic tobacco plants. FEBS Letters, 469, 132–136.
Merle, C., Perret, S., Lacour, T., Jonval, V., Hudaverdian, S., Garrone, R., Ruggiero, F., & Theisen, M. (2002). Hydroxylated human homotrimeric collagen in Agrobacterium tumefaciens mediated transient expression and in tranasgenic tobacco plants. FEBS Letters, 515, 114–118. 172:213–222.
Yano, A., Maeda, F., & Takekoshi, M. (2004). Transgenic tobacco cells producing the human monoclonal antibodies for hepatitis B surface antigen. Journal of Medical Virology, 73, 208–215.
Smith, M. L., Mason, H. S., & Shuler, M. L. (2002). Hepatitis B surface antigen (HBsAg) expression in plant cell culture: kinetics of antigen accumulation in batch culture and its intracellular form. Biotechnology and Bioengineering, 80, 812–822.
He, J., Lai, H., Engle, M., Gorlatov, S., Gruber, C., Steinkellner, H., Diamond, M.S. and Chen, Q. (2014a). Generation and analysis of novel plant-derived antibody-based therapeutic molecules against West Nile virus. PLoS ONE, 9, e93541.
He, J., Peng, L., Lai, H., Hurtado, J., Stahnke, J., & Chen, Q. (2014). A plant-produced antigen elicits potent immune responses against West Nile virus in mice. BioMed Research International, 2014, 1–10.
WHO (World Health Organization). (2003). Annual report on Lymphatic Filariasis 2002, Geneva 2003. http://whqlibdoc.who.int/hq/2003/WHO_CDS_CPE_CEE_2003.38.pdf
Esterre, P., Plichart, C., Sechan, Y., & Nguyen, N. L. (2001). The impact of 34 years of massive DEC chemotherapy on Wuchereria bancrofti infection and transmission: the Maupiti cohort. Tropical Medicine & International Health, 6(3), 190–195.
Melrose, W. D., Durrheim, D. D., & Burgess, G. W. (2004). Update on immunological tests for lymphatic filariasis. Trends in Parasitology, 6, 255.
Lammie, P. J., Weil, G., Noordin, R., Kaliraj, P., Steel, C., et al. (2004). Recombinant antigen based antibody assays for diagnosis and surveillance of lymphatic filariasis—a multicenter trial. Filaria Journal, 3, 9.
Zerpa, N. C., Wide, A., Noda, J., Bermudez, H., Pabon, R., & Noya, O. (2006). Immunogenicity of synthetic peptides derived from Plasmodium falciparum proteins. Experimental Parasitology, 113, 227.
Hewer, R., & Meyer, D. (2007). Envelope-based HIV vaccine peptides as antigens in HIV-1 Immunodiagnostics. International Journal of Biotechnology, 9, 277.
Purcell, A. W., McCluskey, J., & Rossjohn, J. (2007). More than one reason to rethink the use of peptides in vaccine design. Natural, 6, 404.
Pandiaraja, P., Arunkumar, C., Hoti, S. L., Rao, D. N., & Kaliraj, P. (2010). Evaluation of synthetic peptides of WbSXP-1 for the diagnosis of human lymphatic filariasis. Diagnostic Microbiology and Infectious Disease, 68(4), 410–415.
Khoudi, H., Laberrge, S., Ferullo, J. M., Bazin, R., Darveau, A., Castonguay, Y., Allard, G., Lemeieux, R., & Vezina, L. P. (1999). Production of diagnostic monoclonal antibody in perennial alfalfa plants. Biotechnology and Bioengineering, 64, 135–143.
Austin, S., Bingham, E. T., Koegel, R. G., Mathews, D. E., Shahan, M. N., Straub, R. J., & Burgess, R. R. (1994). An overview of a feasibility study for the production of industrial enzymes in transgenic alfalfa. Annals of the New York Academy of Sciences, 721, 234–244.
Mason, H. S., Ball, J., & Schi, J. J. (1996). Expression of Norwalk virus capsid protein in transgenic tobacco and potato and its oral immunogenicity. Proceedings of the National Academy of Sciences of the United States of America, 93, 5335–5340.
Alvarez, M. L., Topal, E., Martin, F., & Cardineau, G. A. (2010). Higher accumulation of F1-V fusion recombinant protein in plants after induction of protein body formation. Plant Molecular Biology, 72, 75–89.
Ghosh, S., Malhotra, P., Lalitha, P. V., Guha-Mukherjee, S., & Chauhan, V. S. (2002). Expression of plasmodium falciparum C-terminal region of merozoite surface protein (PfMSP1 19), a potential malariavaccine candidate in tobacco. Plant Science, 162, 335–343.
Cazzonelli, C. I., & Velten, J. (2006). An in vivo luciferase based Agrobacterium infiltration assay system: implications for post transcriptional gene silencing. Planta, 224, 582–597.
Acknowledgments
This research was funded by a grant (LS-30/2010) from WOS-A section of Department of Science & Technology, Government of India to Mathangi Ganapathy. The authors would like to thank The Director, Centre for Biotechnology, Anna University, Chennai for allowing access to the lab and the infrastructure. Thanks are also due to Dr. N. Subramoniam, Sugarcane Breeding Institute, Coimbatore, India for his expertise and to Mr. Jeyender Sethuraman and Mr. V. Kamalakannan for their help in statistical analysis.
Conflict of Interest
We declare that we have no conflict of interest.
Declaration
All human and animal studies have been approved by the appropriate ethics committee. All persons gave their informed consent prior to their inclusion in the study. Samples used were collected in accordance with US Department of Health and Human Services Human Experimentation Guidelines and the Department of Public Health, Chennai, Tamil Nadu, India. All of the procedures followed were in accordance with the guidelines issued by the Department of Public Health, Government of Tamil Nadu, India, for dealing with human subjects. The protocols adopted are also approved by the institutional review board at the Centre for Biotechnology, Anna University, India.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ganapathy, M., Chakravarthi, M., Charles, S.J. et al. Immunodiagnostic Properties of Wucheraria bancrofti SXP-1, a Potential Filarial Diagnostic Candidate Expressed in Tobacco Plant, Nicotiana tabacum . Appl Biochem Biotechnol 176, 1889–1903 (2015). https://doi.org/10.1007/s12010-015-1685-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1685-0