Abstract
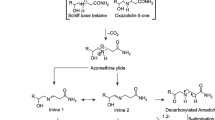
Acrylamide or 2-propenamide is a chemical compound, with chemical formula CH2=CH–CO–NH2, that can be produced at high levels in high-carbohydrate heat-treated foods. The risks of acrylamide to health and its toxic properties (neurotoxicity, genotoxicity, carcinogenicity and reproductive toxicity) were demonstrated by the Scientific Committee on Toxicity, Ecotoxicity and the Environment in 2001. Potato and bakery products account for around 50% and 20% of human exposure to acrylamide, respectively. Factors affecting acrylamide formation and degradation in foods are acrylamide precursors such as free amino acids (mainly asparagine), reducing sugars and processing conditions (i.e. baking time and temperature, moisture content and matrix of product). The aim of this review was to present some results from recent investigations of the effects of different factors affecting acrylamide formation in bakery products. Finally, recommendations are proposed as guidelines for baking manufacturers to reduce the level of acrylamide in their products.





Similar content being viewed by others
References
Ahrné, L., Andersson, C. G., Floberg, P., Rosén, J., & Lingnert, H. (2007). Effect of crust temperature and water content on acrylamide formation during baking of white bread: Steam and falling temperature baking. LWT, 40, 1708–1715.
Aman, P. (1988). The variation in chemical composition of Swedish wheats. Swedish Journal of Agricultural Research, 18, 27–30.
Amrein, T. M., Schönbächler, B., Escher, F., & Amado, R. (2004). Acrylamide in gingerbread: Critical factors for formation and possible ways for reduction. Journal of Agriculture and Food Chemistry, 52, 4282–4288.
Amrein, T. M., Andres, L., Manzardo, G. G., & Amado, R. (2006). Investigations on the promoting effect of ammonium bicarbonate on the formation of acrylamide in model systems. Journal of Agricultural and Food Chemistry, 54, 10253–10261.
Arribas-Lorenzo, G., Fogliano, V., & Morales, F. J. (2009). Acrylamide formation in a cookie system as influenced by the oil phenol profile and degree of oxidation. European Food Research and Technology, 229, 63–72.
Ashoor, S. H., & Zent, J. B. (1984). Maillard browning of common amino acids and sugars. Journal of Food Science, 49, 1206–1207.
Becalski, A., Lau, B. P. Y., Lewis, D., & Seaman, S. W. (2003). Acrylamide in foods: Occurrence, sources, and modelling. Journal of Agriculture and Food Chemistry, 51, 802–808.
Berg, H. E., & Van Boekel, M. A. J. S. (1994). Degradation of lactose during heating of milk, 1. Reaction pathways. Netherlands Milk and Dairy Journal, 48, 157–175.
Biedermann, M., & Grob, K. (2003). Model studies on acrylamide formation in potato, wheat flour and corn starch, ways to reduce acrylamide contents in bakery ware. Mitteilungen aus Lebensmitteluntersuchung und Hygiene, 94, 406–422.
Biedermann, M., Biedermann-Brem, S., Noti, A., Grob, K., Egli, P., & Mandli, H. (2002a). Two GC-MS methods for the analysis of acrylamide in foods. Mitteilungen aus Lebensmitteluntersuchung und Hygiene, 93, 638–652.
Biedermann, M., Noti, A., Biedermann-Brem, S., Mozzetti, V., & Grob, K. (2002b). Experiments on acrylamide formation and possibilities to decrease the potential of acrylamide formation in potatoes. Mitteilungen aus Lebensmitteluntersuchung und Hygiene, 93, 668–687.
Brathen, E., & Knutsen, S. H. (2005). Effect of temperature and time on the formation of acrylamide in starch-based and cereal model systems, flat breads and bread. Food Chemistry, 92(4), 693–700.
Brathen, E., Kita, A., Knutsen, S. H., & Wicklund, T. (2005). Addition of glycine reduces the content of acrylamide in cereal and potato products. Journal of Agricultural and Food Chemistry, 53(8), 3259–3264.
Capuano, E., Oliviero, T., Açar, Ö., Gökmen, V., & Fogliano, V. (2010). Lipid oxidation promotes acrylamide formation in fat-rich model systems. Food Research International, 43, 1021–1026.
Casado, F. J., Sanchez, A. H., & Montano, A. (2010). Reduction of acrylamide content of ripe olives by selected additives. Food Chemistry, 119, 161–166.
Claeys, W. L., De Vleeschouwer, K., & Hendrickx, M. E. (2005). Effect of amino acids on acrylamide formation and elimination kinetics. Biotechnology Progress, 21, 1525–1530.
Claus, A., Mongili, M., Weisz, G., Schieber, A., & Carle, R. (2008). Impact of formulation and technological factors on the acrylamide content of wheat bread and bread rolls. Journal of Cereal Science, 47, 546–554.
Cochran, W. G., & Cox, G. M. (1971). Experimental design (2nd ed.). New York: Wiley.
Curtis, T. Y., Powers, S. J., Balagiannis, D., Elmore, J. S., Mottram, D. S., Parry, M. A. J., et al. (2010). Free amino acids and sugars in rye grain: Implications for acrylamide formation. Journal of Agricultural and Food Chemistry, 58, 1959–1969.
Delatour, T., Périsset, A., Goldmann, T., Riedeker, S., & Stadler, R. H. (2004). Improved sample preparation to determine acrylamide in difficult matrixes such as chocolate powder, cocoa, and coffee by liquid chromatography tandem mass spectrometry. Journal of Agricultural and Food Chemistry, 52, 4625–4631.
Elder, V. A., Fulcher, J. G., Leung, H., & Topor, M. G. (2004). Method for reducing acrylamide in thermally processed foods. Patent US20040058045.
Erbersdobler, H., & Hupe, A. (1991). Determination of lysine damage and calculation of lysine bio-availability in several processed foods. Z. Ernahrugswiss, 30, 46–49.
Eriksson S (2005) Acrylamide in food products: Identification, formation and analytical methodology. PhD thesis. Department of Environmental Chemistry, Stockholm University, Stockholm, Sweden.
Fink, M., Andersson, R., Rosen, J., & Aman, P. (2006). Effect of added asparagine and glycine on acrylamide content in yeast-leavened bread. Cereal Chemistry, 83(2), 218–222.
Fredriksson, H., Tallving, J., Rosen, J., & Aman, P. (2004). Fermentation reduces free asparagine in dough and acrylamide content in bread. Cereal Chemistry, 81(5), 650–653.
Gökmen, V., & Acar, J. (1999). Simultaneous determination of 5-hydroxymethylfurfural and patulin in apple juice by reversed-phase liquid chromatography. Journal of Chromatography A, 847, 69–74.
Gökmen, V., & Senyuva, H. Z. (2007). Acrylamide formation is prevented by divalent cations during the Maillard reaction. Food Chemistry, 103, 196–203.
Gökmen, V., & Şenyuva, H. Z. A. (2006). Simplified approach for the kinetic characterization of acrylamide formation in fructose–asparagine model system. Food Additives and Contaminants, 23, 348–354.
Gökmen, V., Açar, Ö. Ç., Köksel, H., & Acar, J. (2007). Effects of dough formula and baking conditions on acrylamide and hydroxymethylfurfural formation in cookies. Food Chemistry, 104, 1136–1142.
Graf, M., Amrein, T. M., Graf, S., Szalay, R., Escher, F., & Amadò, R. (2006). Reducing the acrylamide content of a semi-finished biscuit on industrial scale. LWT, 39, 724–728.
Grob, K., Biedermann, M., Biedermann-Brem, S., Noti, A., Imhof, D., Amrein, T., et al. (2003). French fries with less than 100 μg/kg acrylamide. A collaboration between cooks and analysis. European Food Research and Technology, 217(3), 185–194.
Haase, N., Matthaeus, B., & Vosmann, K. (2003). Acrylamide in baked products—State of the art. Getreide Mehl und Brot, 57(3), 180–184.
Hagmar, L., Wirfält, E., Paulsoon, B., & Törnqvist, M. (2005). Differences in haemoglobin adduct levels of acrylamide in the general population with respect to dietary intake, smoking habits and gender. Mutation Research, 580, 157–165.
Hamlet, C. G., Baxter, D. E., Sadd, P. A., Slaiding, I., Liang, L., Muller, R., et al. (2005). Exploiting process factors to reduce acrylamide in cereal-based foods, C03032 and C03026. Report prepared on behalf of the U.K. Food Standards Agency, RHM Technology, High Wycombe, UK.
Hamlet, C. G., Sadd, P. A., & Liang, L. (2008). Correlations between the amounts of free asparagine and saccharides present in commercial cereal flours in the UK and the generation of acrylamide during cooking. Journal of Agriculture and Food Chemistry, 56(6145), 6153.
Hedegaard, R. V., Granby, K., Frandsen, H., Thygesen, J., & Skibsted, L. H. (2008). Acrylamide in bread: Effect of prooxidants and antioxidants. European Food Research and Technology, 227, 519–525.
Henle, T., Zehetner, G., & Klostermeyer, H. (1995). Fast and sensitive determination of furosine. Zeitschrift fur Lebensmittel-Untersuchung und Forschung, 200, 235–237.
Hidalgo, F. J., & Zamora, R. (2007). Conversion of phenylalanine into styrene by 2,4-decadienal in model systems. Journal of Agriculture and Food Chemistry, 55, 4902–4906.
Hoenicke, K., & Gatermann, R. (2004). Stability of acrylamide in food during storage. Czech Journal of Food Science, 22, 355–356.
Hoenicke, K., & Gatermann, R. (2005). Studies on the stability of acrylamide in food during storage. Journal of AOAC International, 88, 268–273.
IRAC (International Agency for Research on Cancer). (1994). Some industrial chemicals. IRAC Monographs on the Evaluation of Carcinogenic Risk for Chemicals to Humans, vol. 60 (p. 435). IRAC, Lyon, France.
Johnson, K. A., Gorzinski, S. J., Bodner, K. M., Campbell, R. A., Wolf, C. H., Friedman, M. A., et al. (1986). Chronic toxicity and oncogenicity study on acrylamide incorporated in the drinking water of Fischer 344 rats. Toxicology and Applied Pharmacology, 85, 154.
Jung, M. Y., Choi, D. S., & Ju, J. W. (2003). A novel technique for limitation of acrylamide formation in fried and baked corn chips and in French fries. Journal of Food Science, 68, 1287–1290.
Kita, A., Bråthen, E., Knutsen, S. H., & Wicklund, T. (2005). Effective ways of decreasing acrylamide content in potato crisps during processing. Journal of Agricultural and Food Chemistry, 52, 7011–7016.
Kolek, E., Simko, P., & Simon, P. (2006). Inhibition of acrylamide formation in asparagine/d-glucose model system by NaCl addition. European Food Research and Technology, 224, 283–284.
Kroh, L. W. (1994). Caramelisation in food and beverages. Food Chemistry, 51, 373–379.
Levine, R. A., & Ryan, S. M. (2009). Determining the effect of calcium cations on acrylamide formation in cooked wheat products using a model system. Journal of Agricultural and Food Chemistry, 57, 6823–6829.
Levine, R. A., & Smith, R. E. (2005). Sources of variability of acrylamide levels in cracker model. Journal of Agricultural and Food Chemistry, 53, 4410–4416.
Lindsay, R. C., & Jang, S. (2005). Chemical intervention strategies for substantial suppression of acrylamide formation in fried potato products. Advances in Experimental Medicine and Biology, 561, 393–404.
Mestdagh, F., Maertens, J., Cucu, T., Delporte, K., Van Peteghem, C., & De Meulenaer, B. (2008). Impact of additives to lower the formation of acrylamide in a potato model system through pH reduction and other mechanisms. Food Chemistry, 107, 26–31.
Morales, F. J., Romero, C., & Jiménez-Pérez, S. (1997). Chromatographic determination of bound hydroxymethylfurfural as an index of milk protein glycosylation. Journal of Agricultural and Food Chemistry, 45, 1570–1573.
Mottram, D. S., Wedzicha, B. L., & Dodson, A. T. (2002). Acrylamide is formed in the Maillard reaction. Nature, 419, 448–449.
Mustafa, A., Andersson, R., Rosen, J., Kamal-Eldin, A., & Aman, P. (2005). Factors influencing acrylamide content and color in rye crisp bread. Journal of Agricultural and Food Chemistry, 53, 5985–5989.
Norris, M. V. (1967). Acrylamide. In F. Dee Snell & C. L. Hilton (Eds.), Encyclopedia of industrial chemical analysis, vol. 4 (pp. 160–168). New York: Interscience.
Noti, A., Biedermann-Brem, S., Biedermann, M., Grob, K., Albisser, P., & Realini, P. (2003). Storage of potatoes at low temperature should be avoided to prevent increased acrylamide formation during frying or roasting. Mittelilung Lebensmittel und Hygiene, 94, 167–180.
Press release: HEATOX project completed—brings new pieces to the acrylamide puzzle. 2007. Livsmedelsverket, pp. 9–19.
Ramirez-Jimenez, A. J. (1998). Indicators de las reacciones de pardeamiento químico en productos panarios. Memory of pharmacy degree of licenciate, The University of Granada, Spain.
Ramirez-Jimenez, A. J. (2001). Pardeamiento quimico en derivados de cereals: productos y cereales infantiles. PhD thesis, University of Granada, Spain.
Ramírez-Jiménez, A., Guerra-Hernández, E., & García-Villanova, B. (2000). Browning indicators in bread. Journal of Agriculture and Food Chemistry, 48, 4176–4181.
Ruiz, J. C., Guerra-Hernandez, E., & García-Villanova, B. (2004). Furosine is a useful indicator in pre-baked breads. Journal of the Science of Food and Agriculture, 84, 366–370.
Rydberg, P., Eriksson, S., Tareke, E., Karlsson, P., Ehrenberg, L., & Tornqvist, M. (2003). Investigations of factors that influence the acrylamide content of heated foodstuffs. Journal of Agricultural and Food Chemistry, 51(24), 7012–7018.
Sadd, P. A., Hamlet, G. H., & Liang, L. (2008). Effectiveness of methods for reducing acrylamide in bakery products. Journal of Agricultural and Food Chemistry, 56, 6154–6161.
Samuelson, G. (2003). Acrylamide in food—An update. Scandinavian Journal of Nutrition, 46, 157.
Smith, E. A., & Oehme, F. W. (1991). Acrylamide and polyacrylamide: A review of production, use, environmental fate and neurotoxicity. Reviews on Environmental Health, 9, 215.
Stadler, R. H., Blank, I., Varga, N., Robert, F., Hau, J., Guy, P. A., et al. (2002). Acrylamide from Maillard reaction products. Nature, 419, 449–450.
Summa, C., Wenzl, T., Brohee, M., De La Calle, B., & Anklam, E. (2006). Investigation of the correlation of the acrylamide content and the antioxidant activity of model cookies. Journal of Agricultural and Food Chemistry, 54, 853–859.
Surdyk, N., Rosén, J., Andersson, R., & Åman, P. (2004). Effects of asparagine, fructose and baking conditions on acrylamide content in yeast-leavened wheat bread. Journal of Agricultural and Food Chemistry, 52, 2047–2051.
Svensson, K., Abramsson, L., Becker, W., Glynn, A., Hellenas, K. E., Lind, Y., et al. (2003). Dietary intake of acrylamide in Sweden. Journal of Food Chemistry and Toxicology, 41, 1581–1586.
Taeymans, D., Wood, J., Ashby, P., Blank, I., Studer, A., Stadler, R. H., et al. (2004). A review of acrylamide: An industry perspective on research, analysis, formation, and control. Critical Revue in Food Science and Nutrition, 44, 323–347.
Tareke, E. (2003). Identification and origin of potential background carcinogens: Endogenous isoprene and oxiranes, dietary acrylamide. Doctoral thesis, Department of Environmental Chemistry, Stockholm University, Sweden.
Tareke, E., Rydberg, P., Karlsson, P., Eriksson, S., & Törnqvist, M. (2002). Analysis of acrylamide, a carcinogen formed in heated foodstuffs. Journal of Agricultural and Food Chemistry, 50, 4998–5006.
Tkachuk, R. (1979). Free amino acids in germinated wheat. Journal of the Science of Food and Agriculture, 30, 53–58.
Tomoda, Y., Hanaoka, A., Yasuda, T., Takayama, T., Hiwatashi, A. (2004). US Patent Application 20040126469.
Törqvist, M. (2005). Acrylamide in food: The discovery and its implications. In M. Friedman & D. Mottram (Eds.), Chemistry and safety of acrylamide in food (pp. 1–19). New York: Springer Science +Business Media Inc.
Umano, K., & Shibamoto, T. (1987). Analysis of acrolein from heated cooking oils and beef fat. Journal of Agriculture and Food Chemistry, 35, 909–912.
Vadlamani, K. R., & Seib, P. A. (1999). Effect of zinc and aluminum ions in breadmaking. Cereal Chemistry, 76, 355–360.
Vass, M., Amrein, T. M., Schönbächler, B., Escher, F., & Amadò, R. (2004). Ways to reduce the acrylamide formation in cracker products. Czech Journal of Food Science, 22, 19–21.
Weisshaar, R. (2004). Acrylamide in heated potato products—Analytic and formation routes. European Journal of Lipid Science and Technology, 106, 786–792.
Wenzl, T., Beatriz de la Calle, M., & Anklam, E. (2003). Analytical methods for the determination of acrylamide in food products: A review. Food Additives and Contaminants, 20(10), 885–902.
Yasuhara, A., Tanaka, Y., Hengel, M., & Shibamoto, T. (2003). Gas chromatographic investigation of acrylamide formation in browning model systems. Journal of Agricultural and Food Chemistry, 5(14), 3999–4003.
Yaylayan, V. A., Wnorowski, A., & Perez-Locas, C. (2003). Why asparagines needs carbohydrates to generate acrylamide. Journal of Agriculture and Food Chemistry, 51, 1753–1757.
Zamora, R., Gallardo, E., & Hidalgo, F. J. (2007). Strecker degradation initiated by 2,4-decadienal or methyl 13-oxooctadeca-9,11-dienoate in model systems. Journal of Agriculture and Food Chemistry, 55, 1308–1314.
Zhang, Y., & Zhang, Y. (2007). Study on reduction of acrylamide in fried bread sticks by addition of antioxidant of bamboo leaves and extract of green tea. Asia Pacific Journal of Clinical Nutrition, 16, 131–136.
Zhang, Y., Zhang, G., & Zhang, Y. (2005). Occurrence and analytical methods of acrylamide in heat-treated foods: Review and recent developments. Journal of Chromatography A, 1075, 1–21.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Keramat, J., LeBail, A., Prost, C. et al. Acrylamide in Baking Products: A Review Article. Food Bioprocess Technol 4, 530–543 (2011). https://doi.org/10.1007/s11947-010-0495-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-010-0495-1