Abstract
Purpose of review
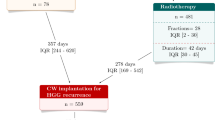
The purpose of this study was to retrospectively evaluate the use of carmustine wafers (CWs) in the management of high-grade gliomas (HGGs). The data from our monoinstitutional series was compared with studies reported in the literature. Special emphasis was placed on the evaluation of side effects and the analysis of extent of resection and molecular profile as risk factors.
Recent findings
The implantation of CWs into the resection cavity during HGG treatment to deliver localized chemotherapy, followed by the Stupp protocol, remains debated in a clinical setting, largely due to the lack of appropriate phase III studies. Given the high expense and poorly characterized side effects associated with CW treatment, identification of patients most likely to benefit from this therapy could be clinically relevant.
Summary
CWs may represent an effective and safe first-line treatment for patients with HGG that exhibit complete tumor resection and harboring a methylated MGMT promoter. Our investigation showed a much larger group of patients exhibiting long-term survival (> = 36 months), strongly supporting a potential survival benefit conferred via CW treatment. The pre-surgical definition of the MGMT promoter status could be of clinical use in identifying “good responders” to CW implantation.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Chowdhary SA, Ryken T, Newton HB. Survival outcomes and safety of carmustine wafers in the treatment of high-grade gliomas: a meta-analysis. J Neurooncol. 2015;122(2):367–82. https://doi.org/10.1007/s11060-015-1724-2.
Stupp R, Mason WP, van den Bent MJ, European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. https://doi.org/10.1056/NEJMoa043330.
Stupp R, Brada M, van den Bent MJ, ESMO Guidelines Working Group et al.. High-grade glioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014; Suppl 3: 93–101.
Stupp R, Hegi ME, Gorlia T, European Organisation for Research and Treatment of Cancer (EORTC).; Canadian Brain Tumor Consortium.; CENTRIC study team, et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071-22072 study): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014;10:1100–8.
Sanai N, Berger MS. Extent of resection influences outcomes for patients with gliomas. Rev. Neurol. 2011;167(10):648–54. https://doi.org/10.1016/j.neurol.2011.07.004.
Grabowski MM, Recinos PF, Nowacki AS, Schroeder JL, Angelov L, Barnett GH, et al. Residual tumor volume versus extent of resection: predictors of survival after surgery for glioblastoma. J Neurosurgery. 2014;121(5):1115–23. https://doi.org/10.3171/2014.7.JNS132449.
McGirt MJ, Than KD, Weingart JD, Chaichana KL, Attenello FJ, Olivi A, et al. Gliadel (BCNU) wafer plus concomitant temozolomide therapy after primary resection of glioblastoma multiforme. J Neurosurg. 2009;110(3):583–8. https://doi.org/10.3171/2008.5.17557.
Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. J Neurosurg. 2001;95(2):190–8. https://doi.org/10.3171/jns.2001.95.2.0190.
Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460–9. https://doi.org/10.1001/jamaoncol.2016.1373.
Affronti ML, Heery CR, Herndon JE 2nd, et al. Overall survival of newly diagnosed glioblastoma patients receiving carmustine wafers followed by radiation and concurrent temozolomide plus rotational multiagent chemotherapy. Cancer. 2009;115(15):3501–11. https://doi.org/10.1002/cncr.24398.
Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):709–22. https://doi.org/10.1056/NEJMoa1308345.
Gilbert MR, Dignam JJ, Armstrong TS, Wefel JS, Blumenthal DT, Vogelbaum MA, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370(8):699–708. https://doi.org/10.1056/NEJMoa1308573.
Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, et al. Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol. 2013;31(32):4085–91. https://doi.org/10.1200/JCO.2013.49.6968.
Batchelor TT, Mulholland P, Neyns B, Nabors LB, Campone M, Wick A, et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol. 2013;31(26):3212–8. https://doi.org/10.1200/JCO.2012.47.2464.
Clarke JL, Molinaro AM, Phillips JJ, Butowski NA, Chang SM, Perry A, et al. A single-institution phase II trial of radiation, temozolomide, erlotinib, and bevacizumab for initial treatment of glioblastoma. Neuro Oncol. 2014;16(7):984–90. https://doi.org/10.1093/neuonc/nou029.
Westphal M, Heese O, Steinbach JP, Schnell O, Schackert G, Mehdorn M, et al. A randomised, open label phase III trial with nimotuzumab, an anti-epidermal growth factor receptor monoclonal antibody in the treatment of newly diagnosed adult glioblastoma. Eur J Cancer. 2015;51(4):522–32. https://doi.org/10.1016/j.ejca.2014.12.019.
Chamberlain MC. Radiographic patterns of relapse in glioblastoma. J Neurooncol. 2011;101(2):319–23. https://doi.org/10.1007/s11060-010-0251-4.
Xing WK, Shao C, Qi ZY, et al. The role of Gliadel wafers in the treatment of newly diagnosed GBM: a meta-analysis. Drug Des Devel Ther. 2015;9:3341–8.
• Ashby LS, Smith KA, Stea B. Gliadel wafer implantation combined with standard radiotherapy and concurrent followed by adjuvant temozolomide for treatment of newly diagnosed high-grade glioma: a systematic literature review. World J Surg Oncol. 2016;14:225. A recent meta-analysis of the largest retrospective trials of CWs plus RT/TMZ in HGG patients.
Bregy A, Shah AH, Diaz MV, Pierce HE, Ames PL, Diaz D, et al. The role of Gliadel wafers in the treatment of high-grade gliomas. Expert Rev. Anticancer Ther. 2013;13(12):1453–61. https://doi.org/10.1586/14737140.2013.840090.
Watts C, Dunn L, Ashkan K, et al. Establishing the efficacy of Gliadel wafers: progress towards a phase III trial. Acta Neurochir (Wien). 2013;155(1):61–2. https://doi.org/10.1007/s00701-012-1533-8.
• Zhang YD, Dai RY, Chen Z, et al. Efficacy and safety of carmustine wafers in the treatment of glioblastoma multiforme: a systematic review. Turk Neurosurg. 2014;24:639–45. The author performed a systematic review of CWs for the treatment of HGGS to assess the survival benefit and safety of this therapy
Gutenberg A, Lumenta CB, Braunsdorf WE, et al. The combination of carmustine wafers and temozolomide for the treatment of malignant gliomas. A comprehensive review of the rationale and clinical experience. J Neurooncol. 2013;113(2):163–74. https://doi.org/10.1007/s11060-013-1110-x.
•• Pallud J, Audureau E, Noel G, et al. Long-term results of carmustine wafer implantation for newly diagnosed glioblastomas: a controlled propensity-matched analysis of a French multicenter cohort. Neuro Oncol. 2015;17:1609–19. This study is the first multicenter study with a large cohort in the use of CWs implantation in HGGs newly diagnosed.
•• Roux A, Peeters S, Zanello M, Bou Nassif R, et al. Extent of resection and carmustine wafer implantation safely improve survival in patients with a newly diagnosed glioblastoma: a single center experience of the current practice. J Neurooncol. 2017;135:83–92. In this single center-study the authors compared HGGs treated with and without CWs implantation, and demonstrated that CW implantation in combination with maximal resection, followed by standard combined chemoradiotherapy is safe, efficient, and well-tolerated.
Brem H, Ewend MG, Piantadosi S, et al. The safety of interstitial chemotherapy with BCNU-loaded polymer followed by radiation therapy in the treatment of newly diagnosed malignant gliomas: phase I trial. J Neuroncol. 1995;26:111–23.
Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. Lancet. 1995;345:1008–12.
Valtonen S, Timonen U, Toivanen P, et al. Interstitial chemotherapy with carmustine-loaded polymers for high-grade gliomas: a randomized double-blind study. Neurosurgery. 1997;41:44–8.
Westphal M, Hilt DC, Bortey E, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88.
Smith KA, Ashby LS, Gonzalez LF, et al. Prospective gross total resection with Gliadel wafers followed by early postoperative Gamma Knife radiosurgery and conformal fractioned radiotherapy as the initial treatment for patients with radiographically suspected newly diagnosed glioblastoma multiforme. J Neurosurg. 2008;109(Suppl):106–17.
Duntze J, Litré CF, Eap C, et al. Implanted carmustine wafers followed by concomitant radiochemotherapy to treat newly diagnosed malignant gliomas: prospective, observational, multicenter study on 92 cases. Ann Surg Oncol. 2013;20:2065–72.
Barr JG, Grundy PL. The effects of the NICE Technology Appraisal 121 (gliadel and temozolomide) on survival in high-grade glioma. Br J Neurosurg. 2012;26:818–22.
Burri SH, Prabhu RS, Sumrall AL, et al. BCNU wafer placement with temozolomide (TMZ) in the immediate postoperative period after tumor resection followed by radiation therapy with TMZ in patients with newly diagnosed high grade glioma: final results of a prospective, multi-institutional, phase II trial. J Neurooncol. 2015;123:259–66.
Chaichana KL, Zaidi H, Pendleton C, et al. The efficacy of carmustine wafers for older patients with glioblastoma multiforme: prolonging survival. Neurol Res. 2011;33:759–64.
Grossman R, Burger P, Soudry E, et al. MGMT inactivation and clinical response in newly diagnosed GBM patients treated with Gliadel. J Clin Neurosci. 2015;22:1938–42.
•• Lechapt-Zalcman E, Levallet G, Dugué AE, et al. O(6)-Methylguanine-DNA methyltransferase (MGMT) promoter methylation and low MGMT-encoded protein expression as prognostic markers in glioblastoma patients treated with biodegradable carmustine wafer implants after initial surgery followed by radiotherapy with concomitant and adjuvant temozolomide. Cancer. 2012;118:4545–54. The relationship between the MGMT methylation status and clinical outcomes in newly diagnosed glioblastoma multiforme patients who were treated with CWs were investigated, supporting the use of CWs in HGGs.
Miglierini P, Bouchekoua M, Rousseau B, et al. Impact of the per-operatory application of GLIADEL wafers (BCNU, carmustine) in combination with temozolomide and radiotherapy in patients with glioblastoma multiforme: efficacy and toxicity. Clin Neurol Neurosurg. 2012;114:1222–5.
Noël G, Schott R, Froelich S, et al. Retrospective comparison of chemoradiotherapy followed by adjuvant chemotherapy, with or without prior gliadel implantation (carmustine) after initial surgery in patients with newly diagnosed high-grade gliomas. Int J Radiat Oncol Biol Phys. 2012;82:749–55.
Pan E, Mitchell SB, Tsai JS. A retrospective study of the safety of BCNU wafers with concurrent temozolomide and radiotherapy and adjuvant temozolomide for newly diagnosed glioblastoma patients. J Neurooncol. 2008;88:353–7.
Pavlov V, Page P, Abi-Lahoud G, et al. Combining intraoperative carmustine wafers and Stupp regimen in multimodal first-line treatment of primary glioblastomas. Br J Neurosurg. 2015;29:524–31.
Salvati M, D’elia A, Frati A, et al. Safety and feasibility of the adjunct of local chemotherapy with biodegradable carmustine (BCNU) wafers to the standard multimodal approach to high grade gliomas at first diagnosis. J Neurosurg Sci. 2011;55:1–6.
Salmaggi A, Milanesi I, Silvani A, et al. Prospective study of carmustine wafers in combination with 6-month metronomic temozolomide and radiation therapy in newly diagnosed glioblastoma: preliminary results. J Neurosurg. 2013;118:821–9.
Attenello FJ, Mukherjee D, Datoo G, et al. Use of Gliadel (BCNU) wafer in the surgical treatment of malignant glioma: a 10-year institutional experience. Ann Surg Oncol. 2008;1510:2887–93.
Bock HC, Puchner MJ, Lohmann F, et al. First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: a multicenter experience. Neurosurg Rev. 2010;33:441–9.
Nachbichler SB, Schupp G, Ballhausen H, et al. Temozolomide during radiotherapy of glioblastoma multiforme: daily administration improves survival. Strahlenther Onkol. 2017;193:890–6.
De Bonis P, Fiorentino A, Anile C, et al. The impact of repeated surgery and adjuvant therapy on survival for patients with recurrent glioblastoma. Clin Neurol Neurosurg. 2013;115:883–6.
Keles GE, Anderson B, Berger MS. The effect of extent of resection on time to tumor progression and survival in patients with glioblastoma multiforme of the cerebral hemisphere. Surg Neurol. 1999;52:371–9.
Li H, Li J, Cheng G, Zhang J, Li X. IDH mutation and MGMT promoter methylation are associated with the pseudoprogression and improved prognosis of glioblastoma multiforme patients who have undergone concurrent and adjuvant temozolomide-based chemoradiotherapy. Clin Neurol Neurosurg. 2016;151:31–6.
Millward CP, Brodbelt AR, Haylock B, et al. The impact of MGMT methylation and IDH-1 mutation on long-term outcome for glioblastoma treated with chemoradiotherapy. Acta Neurochir (Wien). 2016;158:1943–53.
Juratli TA, Kirsch M, Geiger K, et al. The prognostic value of IDH mutations and MGMT promoter status in secondary high-grade gliomas. J Neurooncol. 2012;110:325–33.
Ius T, Isola M, Budai R, et al. Low-grade glioma surgery in eloquent areas: volumetric analysis of extent of resection and its impact on overall survival. A single-institution experience in 190 patients. J Neurosurg. 2012;117:1039–52.
Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131:803–20.
Preusser M, Berghoff AS, Manzl C, et al. Clinical Neuropathology practice news 1-2014: pyrosequencing meets clinical and analytical performance criteria for routine testing of MGMT promoter methylation status in glioblastoma. Clin Neuropathol. 2014;33:6–14.
Smith JS, Chang EF, Lamborn KR, et al. Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol. 2008;26:1338–45.
Steyerberg EW. Clinical prediction models: a practical approach to development, validation, and updating. New York: Springer; 2009.
•• Fiano V, Trevisan M, Trevisan E, et al. Mgmt promoter methylation in plasma of glioma patients receiving temozolomide. J Neurooncol. 2014;117:347–57. In this study, the value of MGMT promoter methylation status in plasma as a prognostic/predictive biomarker in glioma patients was assessed
Wang Z, Jiang W, Wang Y, et al. Mgmt promoter methylation in serum and cerebrospinal fluid as a tumor-specific biomarker of glioma. Biomed Rep. 2015;3:543–8.
Weaver KD, Grossman SA, Herman JG. Methylated tumor-specific DNA as a plasma biomarker in patients with glioma. Cancer Invest. 2006;24:35–40.
Acknowledgements
We thank Dr. Stefano Pizzolitto and Dr. Giovanna De Maglio for their invaluable support.
Funding
FIRB accordi di programma 2011 pr. RBAP11ETKA_007 “Nanotechnological approaches for tumor theragnostic” (APB). Programma per la Cooperazione Transfrontaliera Italia-Slovenia 2007–2013. Title: “Identificazione di nuovi marcatori di cellule staminali tumorali a scopo diagnostico e terapeutico” (MS, CDL). Project ERC- 7FP SP 2 IDEAS QUIDPROQUO G.A. n. 269051. Title: “Molecular nanotechnology for life science applications: quantitative interactomics for diagnostics, proteomics and quantitative oncology” (DC).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
Ius, T., Cesselli, D., Isola, M. et al. Combining Clinical and Molecular Data to Predict the Benefits of Carmustine Wafers in Newly Diagnosed High-Grade Gliomas. Curr Treat Options Neurol 20, 3 (2018). https://doi.org/10.1007/s11940-018-0489-2
Published:
DOI: https://doi.org/10.1007/s11940-018-0489-2