Abstract
Purpose of Review
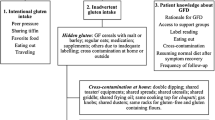
Gluten is a commonly ingested polymeric protein found in wheat, barley, and rye that has gained recent notoriety because of its relationship to disease and health. Avoidance of gluten is appropriate in patients with a diagnosed gluten–related disorder and may have treatment implications in other diseases of the digestive tract. This review highlights current knowledge of gluten related disorders and the use of a gluten-free diet in gastrointestinal disease management.
Recent Findings
Gluten-free diets should be used in patients with a diagnosed gluten–related disorder including celiac disease, non-celiac gluten sensitivity, and wheat-sensitive eosinophilic esophagitis. Use of this diet in management of other digestive conditions including gastroesophageal reflux disease, irritable bowel syndrome, and inflammatory bowel disease is controversial and not currently supported by the literature.
Summary
This review provides a framework for classifying gluten-related disorders in terms of pathogenesis, understanding the literature that supports dietary avoidance in modulation of gastrointestinal disease, and identifies limitations of dietary restriction in patients.

Similar content being viewed by others
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Guandalini S, Gokhale R. Update on immunologic basis of celiac disease. Curr Opin Gastroenterol. 2002;18(1):95–100 Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&NEWS=n&CSC=Y&PAGE=fulltext&D=ovft&AN=00001574-200201000-00017.
Kim H, Patel KG, Orosz E, et al. Time trends in the prevalence of celiac disease and gluten-free diet in the US population: results from the national health and nutrition examination surveys 2009-2014. JAMA Int Med. 2016;176(11):1716–7. Available from. https://doi.org/10.1001/jamainternmed.2016.5254.
Stamnaes J, Sollid LM. Celiac disease: autoimmunity in response to food antigen. Semin Immunol. 2015;27(5):343–52 Available from: https://www.clinicalkey.es/playcontent/1-s2.0-S104453231500072X.
Cebolla Á, Moreno ML, Coto L, Sousa C. Gluten immunogenic peptides as standard for the evaluation of potential harmful prolamin content in food and human specimen. Nutrients. 2018;10(12):1927 Available from: https://www.ncbi.nlm.nih.gov/pubmed/30563126.
Real A, Comino I, de Lorenzo L, et al. Molecular and immunological characterization of gluten proteins isolated from oat cultivars that differ in toxicity for celiac disease. PLoS One. 2012;7(12):e48365 Available from: https://www.ncbi.nlm.nih.gov/pubmed/23284616.
Graveland A, Bosveld P, Lichtendonk WJ, et al. A model for the molecular structure of the glutenins from wheat flour. J Cereal Sci. 1985;3(1):1–16 Available from: https://www.sciencedirect.com/science/article/pii/S0733521085800291.
Kagnoff MF. Celiac Disease Pathogenesis of a model immunogenetic disease. J Clin Invest. 2007;117(1):41–9 Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1716218/.
Pietzak M. Celiac disease, wheat allergy, and gluten sensitivity. J Parenter Enteral Nutr. 2012;36(1_suppl):75S Available from: https://journals.sagepub.com/doi/full/10.1177/0148607111426276.
•• Sapone A, Bai J, Ciacci C, et al. Spectrum of gluten-related disorders: consensus on new nomenclature and classification. BMC Medicine. 2012;10:13 Available from: https://search.datacite.org/works/10.7916/D8PR7TBW. This consensus statement defines current classification of gluten related disorders based on pathogenesis of disease.
Cianferoni A. Wheat allergy: diagnosis and management. J Asthma Allergy. 2016;9:13–25 Available from: https://www.ncbi.nlm.nih.gov/pubmed/26889090.
Reed CC, Dellon ES. Eosinophilic esophagitis. Med Clin of N Amer. 2019;103(1):29–42 Available from: https://www.sciencedirect.com/science/article/pii/S0025712518301007.
Leonard MM, Sapone A, Catassi C, et al. Celiac disease and nonceliac gluten sensitivity: A review. JAMA. 2017;318(7):647–56. Available from. https://doi.org/10.1001/jama.2017.9730.
Kim SM, Mayassi T, Jabri B. Innate immunity: actuating the gears of celiac disease pathogenesis. Best Prac Res: Clin Gastroenterol. 2015;29(3):425–35 Available from: https://www.clinicalkey.es/playcontent/1-s2.0-S1521691815000554.
Bolotin D, Petronic-Rosic V. Dermatitis herpetiformis. J Am Acad Dermatol. 2010;64(6):1017–24 Available from: https://www.clinicalkey.es/playcontent/1-s2.0-S0190962210021225.
Rubio-Tapia A, Hill ID, Kelly CP, et al. ACG clinical guidelines: diagnosis and management of celiac disease. Amer J Gastroenterol. 2013;108(5):656–76 Available from: https://www.ncbi.nlm.nih.gov/pubmed/23609613.
Hadjivassiliou M, Boscolo S, Davies-Jones GAB, et al. The humoral response in the pathogenesis of gluten ataxia. Neurology. 2002;58(8):1221–6 Available from: https://www.ncbi.nlm.nih.gov/pubmed/11971090.
Hadjivassiliou M, Davies-Jones GAB, Sanders DS, et al. Dietary treatment of gluten ataxia. J Neurol, Neurosurg & Psych. 2003;74(9):1221–4. Available from. https://doi.org/10.1136/jnnp.74.9.1221.
Hollon J, Puppa EL, Greenwald B, et al. Effect of gliadin on permeability of intestinal biopsy explants from celiac disease patients and patients with non-celiac gluten sensitivity. Nutrients. 2015;7(3):1565–76 Available from: https://www.ncbi.nlm.nih.gov/pubmed/25734566.
Matuchansky C. Gluten-free diet and irritable bowel syndrome–diarrhea. Gastroenterol. 2013;145(3):692–3 Available from: https://www.clinicalkey.es/playcontent/1-s2.0-S0016508513009992.
Roque V, Camilleri MI, Smyrk M, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterol. 2013;144(5):911.e3 Available from: https://www.clinicalkey.es/playcontent/1-s2.0-S0016508513001352.
Lebwohl B, Ludvigsson J, Green PH. Celiac disease and non-celiac gluten sensitivity. BMJ. 2015;351:h4347 Available from: htps://www.bmj.com/content/351/bmj.h4347.
Bardella MT, Fredella C, Prampolini L. Body composition and dietary intakes in adult celiac disease patients consuming a strict gluten-free diet. Amer J Clini Nutr. 2000;72(4):937–9 Available from: https://www.ncbi.nlm.nih.gov/pubmed/11010934.
Kupper C. Dietary guidelines and implementation for celiac disease. Gastroenterol. 2005;128(4):S127 Available from: https://www.sciencedirect.com/science/article/pii/S0016508505001939.
Nachman F, Sugai E, Vázquez H, et al. Serological tests for celiac disease as indicators of long-term compliance with the gluten-free diet. Eur J Gastroenterol Hepatol. 2011;23(6):473–80 Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&NEWS=n&CSC=Y&PAGE=fulltext&D=ovft&AN=00042737-201106000-00005.
Malamut G, Murray J, Cellier C. Refractory celiac disease. Gastrointest Endosc Clin N Am. 2012;22(4):759–72 Available from: https://www.clinicalkey.es/playcontent/1-s2.0-S1052515712000992.
Cash BD, Rubenstein JH, Young PE, et al. The prevalence of celiac disease among patients with non-constipated irritable bowel syndrome is similar to controls. Gastroenterology. 2011;141(4):1187–93 Available from: https://www.ncbi.nlm.nih.gov/pubmed/21762658.
•• Biesiekierski JR, Peters SL, Newnham ED, et al. No effects of gluten in patients with self-reported non-celiac gluten sensitivity after dietary reduction of fermentable, poorly absorbed, short-chain carbohydrates. Gastroenterol. 2013;145(2):328.e3 Available from: https://www.clinicalkey.es/playcontent/1-s2.0-S0016508513007026. This article discusses the potential overlap between symptoms induced by gluten and FODMAP ingestion by double blind crossover trial.
Dellon ES, Gonsalves N, Hirano I. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Amer J Gastroenterol. 2013;108(5):679–92 Available from: https://www.ncbi.nlm.nih.gov/pubmed/23567357.
Warners MJ, Vlieg-Boerstra BJ, Bredenoord AJ. Elimination and elemental diet therapy in eosinophilic oesophagitis. Best Prac Res: Clin Gastroenterol. 2015;29(5):793–803 Available from: https://www.clinicalkey.es/playcontent/1-s2.0-S1521691815000980.
Kagalwalla AF, Sentongo TA, Ritz S. Effect of six-food elimination diet on clinical and histologic outcomes in eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2006;4(9):1097–102 Available from:.
Molina-Infante J, Lucendo AJ. Dietary therapy for eosinophilic esophagitis. J Allergy Clin Immunol. 2018;142(1):41–7 Available from: https://www.sciencedirect.com/science/article/pii/S0091674918303208.
Gonsalves N, Yang G-Y, Doerfler B, et al. Elimination diet effectively treats eosinophilic esophagitis in adults; food reintroduction identifies causative factors. Gastroenterol. 2012;142(7):1459.e1 Available from: https://www.clinicalkey.es/playcontent/1-s2.0-S0016508512003095.
Kagalwalla A, Shah A, Li B, et al. Identification of specific foods responsible for inflammation in children with eosinophilic esophagitis successfully treated with empiric elimination diet. J Ped Gastroenterol Nut. 2011;53(2):145–9 Available from: http://ovidsp.ovid.com/ovidweb.cgi?T=JS&NEWS=n&CSC=Y&PAGE=fulltext&D=ovft&AN=00005176-201108000-00006.
Doerfler B, Bryce P, Hirano I, et al. Practical approach to implementing dietary therapy in adults with eosinophilic esophagitis: the chicago experience. Dis Esophagus. 2015;28(1):42–58 Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/dote.12175.
Kliewer K, Venter C, Cassin A, et al. Should wheat, barley, rye, and/or gluten be avoided in a 6-food elimination diet? J Allergy Clin Immunol. 2015;137(4):1011–4 Available from: https://www.clinicalkey.es/playcontent/1-s2.0-S0091674915017261.
Vakil N, van Zanten Sander V, Kahrilas P, et al. The montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. Amer J Gastroenterol. 2006;101(8):1900–20. Available from. https://doi.org/10.1111/j.1572-0241.2006.00630.x.
Ness-Jensen E, Hveem K, El-Serag H, et al. Lifestyle intervention in gastroesophageal reflux disease. Clin Gastroenterol Hepatol. 2016;14(2):175 Available from: http://kipublications.ki.se/Default.aspx?queryparsed=id:132991735.
MacFarlane NG. Digestion and absorption. Anesth Int Care Med. 2018;19(3):125–7 Available from: https://www.sciencedirect.com/science/article/pii/S1472029918300018.
Piche T, des Varannes SB, Sacher-Huvelin S, Holst JJ, et al. Colonic fermentation influences lower esophageal sphincter function in gastroesophageal reflux disease. Gastroenterol. 2003;124(4):894–902 Available from: https://www.sciencedirect.com/science/article/pii/S0016508503000738.
Morozov S, Isakov V, Konovalova M. Fiber-enriched diet helps to control symptoms and improves esophageal motility in patients with non-erosive gastroesophageal reflux disease. World J Gastroenterol. 2018;24(21):2291–9 Available from: https://www.ncbi.nlm.nih.gov/pubmed/29881238.
DiSilvestro RA, Verbruggen MA, Offutt EJ. Anti-heartburn effects of a fenugreek fiber product. Phytotherapy Res. 2011;25(1):88–91 Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/ptr.3229.
Usai P, Manca R, Cuomo R, et al. Effect of gluten-free diet on preventing recurrence of gastroesophageal reflux disease–related symptoms in adult celiac patients with nonerosive reflux disease. J Gastroenterol Hepatol. 2008;23(9):1368–72 Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1440-1746.2008.05507.x.
Zhang Y-Z, Li Y-Y. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014;20(1):91–9 Available from: https://www.wjgnet.com/1007-9327/full/v20/i1/91.htm.
Hou JK, Bincy AB, El-Serag H. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol. 2011;106(4):563–73 Available from: https://www.ncbi.nlm.nih.gov/pubmed/21468064.
Limketkai BN, Iheozor-Ejiofor Z, Gjuladin-Hellon T, et al. Dietary interventions for induction and maintenance of remission in inflammatory bowel disease. Cochrane Database Syst Rev. 2019;2:CD012839 Available from: https://www.ncbi.nlm.nih.gov/pubmed/30736095.
Herfarth HH, Martin CF, Sandler RS, et al. Prevalence of a gluten-free diet and improvement of clinical symptoms in patients with inflammatory bowel diseases. Inflamm Bowel Dis. 2014;20(7):1994–7 Available from: https://www.ncbi.nlm.nih.gov/pubmed/24865778.
Limketkai BN, Sepulveda R, Hing T, et al. Prevalence and factors associated with gluten sensitivity in inflammatory bowel disease. Scand J Gastroenterol. 2018;53(2):147–51 Available from: http://www.tandfonline.com/doi/abs/10.1080/00365521.2017.1409364.
Singh J, Whelan K. Limited availability and higher cost of gluten-free foods. J Human Nutr Diet. 2011;24(5):479–86 Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-277X.2011.01160.x.
Allen B, Orfila C. The availability and nutritional adequacy of gluten-free bread and pasta. Nutrients. 2018;10(10):1370 Available from: https://www.ncbi.nlm.nih.gov/pubmed/30257431.
Shepherd SJ, Gibson PR. Nutritional inadequacies of the gluten-free diet in both recently-diagnosed and long-term patients with coeliac disease. J Hum Nutr Diet. 2013;26(4):349–58 Available from: https://onlinelibrary.wiley.com/doi/abs/10.1111/jhn.12018.
Vici G, Belli L, Biondi M, et al. Gluten free diet and nutrient deficiencies: a review. Clin Nutr. 2016;35(6):1236–41 Available from: https://www.clinicalkey.es/playcontent/1-s2.0-S0261561416300887.
Thompson T. Folate, Iron and dietary fiber contents of the GFD. J Amer Diet Assoc. 2000;100(11):1389–96 Available from: https://www.researchgate.net/publication/12223715_Folate_Iron_and_Dietary_Fiber_Contents_of_the_Gluten-free_Diet.
Thompson T. Thiamine, riboflavin, and niacin contents of the GFD. J Am Diet Assoc. 1999;99(7):858–62 Available from: https://linkinghub.elsevier.com/retrieve/pii/S0002822399002059.
Newberry C, McKnight L, Sarav M, et al. Going gluten free: the history and nutritional implications of today’s most popular diet. Curr Gastroenterol Rep. 2017;19(11):1–8 Available from: https://www.ncbi.nlm.nih.gov/pubmed/28948465.
Author information
Authors and Affiliations
Contributions
Carolyn Newberry, MD was involved in the manuscript research, preparation, and final approval of the manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Carolyn Newberry declares the she has no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Obesity and Nutrition
Rights and permissions
About this article
Cite this article
Newberry, C. The Gluten-Free Diet: Use in Digestive Disease Management . Curr Treat Options Gastro 17, 554–563 (2019). https://doi.org/10.1007/s11938-019-00255-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-019-00255-0