Abstract
Purpose of Review
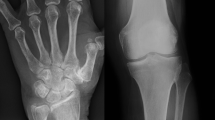
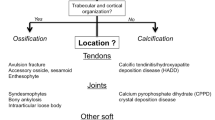
Calcium pyrophosphate deposition disease (CPPD) arises from calcium pyrophosphate deposition throughout the body, leading to different clinical syndromes that may be diagnosed using various imaging modalities. The purpose of this review is to highlight recent updates in the imaging of CPPD.
Recent Findings
Conventional radiography remains the initial test when imaging CPPD; but musculoskeletal ultrasound and conventional computed tomography (CT) may also assist in diagnosing and characterizing CPP deposits, with increased sensitivity. Dual-energy CT is also being used to differentiate CPP crystals from other crystal deposition diseases. CPP discitis has been diagnosed with MRI, but MRI has lower sensitivity and specificity than the aforementioned imaging studies in CPPD diagnosis.
Summary
Assorted imaging modalities are increasingly used to diagnose CPPD involving atypical joints, avoiding invasive procedures. Each modality has its advantages and disadvantages. Future imaging may be able to provide more utility than what is currently available.
Similar content being viewed by others
References
Papers of particular interest, published recently have been highlighted as: • Of importance • • Of major importance
Miksanek J, Rosenthal AK. Imaging of calcium pyrophosphate deposition disease. Curr Rheumatol Rep. 2015;17(3):20. https://doi.org/10.1007/s11926-015-0496-1.
Zhang W, Doherty M, Bardin T, Barskova V, Guerne PA, Jansen TL, et al. European League Against Rheumatism recommendations for calcium pyrophosphate deposition. Part I: terminology and diagnosis. Ann Rheum Dis. 2011;70(4):563–70. https://doi.org/10.1136/ard.2010.139105.
Rosenthal AK, Ryan LM. Calcium pyrophosphate deposition disease. N Engl J Med. 2016;374(26):2575–84. https://doi.org/10.1056/NEJMra1511117.
Segal JB, Albert D. Diagnosis of crystal-induced arthritis by synovial fluid examination for crystals: lessons from an imperfect test. Arthritis Care Res. 1999;12(6):376–80. https://doi.org/10.1002/1529-0131(199912)12:6%3c376::aid-art5%3e3.0.co;2-5.
Announ N, Guerne PA. [Diagnosis and treatment of calcium pyrophosphate crystal-induced arthropathy]. Z Rheumatol. 2007;66(7):573–4, 6–8. https://doi.org/10.1007/s00393-007-0221-1.
Filippou G, Adinolfi A, Cimmino MA, Scirè CA, Carta S, Lorenzini S, et al. Diagnostic accuracy of ultrasound, conventional radiography and synovial fluid analysis in the diagnosis of calcium pyrophosphate dihydrate crystal deposition disease. Clin Exp Rheumatol. 2016;34(2):254–60.
Forien M, Combier A, Gardette A, Palazzo E, Dieudé P, Ottaviani S. Comparison of ultrasonography and radiography of the wrist for diagnosis of calcium pyrophosphate deposition. Joint Bone Spine. 2018;85(5):615–8. https://doi.org/10.1016/j.jbspin.2017.09.006.
O'Neill J, (ed). Essential imaging in rheumatology. Crystal-Related Disease. New York: Springer Science+Business Media; 2015.
Resnick D, Niwayama G, Goergen TG, Utsinger PD, Shapiro RF, Haselwood DH, et al. Clinical, radiographic and pathologic abnormalities in calcium pyrophosphate dihydrate deposition disease (CPPD): pseudogout. Radiology. 1977;122(1):1–15. https://doi.org/10.1148/122.1.1.
• Cho NH, Song Y, Lee S, Sung YK, Jun JB. Incidence of knee chondrocalcinosis and its risk factors in a community-based cohort. Int J Rheum Dis. 2018;21(7):1391–7. https://doi.org/10.1111/1756-185x.13317. This was a large epidemiologic study that showed the incidence of knee chondrocalcinosis.
Musacchio E, Ramonda R, Perissinotto E, Sartori L, Hirsch R, Punzi L, et al. The impact of knee and hip chondrocalcinosis on disability in older people: the ProVA Study from northeastern Italy. Ann Rheum Dis. 2011;70(11):1937–43. https://doi.org/10.1136/ard.2011.150508.
Falkowski AL, Jacobson JA, Kalia V, Meyer NB, Gandikota G, Yosef M, et al. Cartilage icing and chondrocalcinosis on knee radiographs in the differentiation between gout and calcium pyrophosphate deposition. PLoS ONE. 2020;15(4):e0231508. https://doi.org/10.1371/journal.pone.0231508.
Abhishek A, Doherty S, Maciewicz R, Muir K, Zhang W, Doherty M. Chondrocalcinosis is common in the absence of knee involvement. Arthritis Res Ther. 2012;14(5):R205. https://doi.org/10.1186/ar4043.
Frediani B, Filippou G, Falsetti P, Lorenzini S, Baldi F, Acciai C, et al. Diagnosis of calcium pyrophosphate dihydrate crystal deposition disease: ultrasonographic criteria proposed. Ann Rheum Dis. 2005;64(4):638–40. https://doi.org/10.1136/ard.2004.024109.
Lee KA, Lee SH, Kim HR. Diagnostic value of ultrasound in calcium pyrophosphate deposition disease of the knee joint. Osteoarthritis Cartilage. 2019;27(5):781–7. https://doi.org/10.1016/j.joca.2018.11.013.
Cipolletta E, Filippou G, Scirè CA, Di Matteo A, Di Battista J, Salaffi F, et al. The diagnostic value of conventional radiography and musculoskeletal ultrasonography in calcium pyrophosphate deposition disease: a systematic literature review and meta-analysis. Osteoarthritis Cartilage. 2021. https://doi.org/10.1016/j.joca.2021.01.007.
Patil P, Dasgupta B. Role of diagnostic ultrasound in the assessment of musculoskeletal diseases. Ther Adv Musculoskelet Dis. 2012;4(5):341–55. https://doi.org/10.1177/1759720x12442112.
Aouba A, Vuillemin-Bodaghi V, Mutschler C, De Bandt M. Crowned dens syndrome misdiagnosed as polymyalgia rheumatica, giant cell arteritis, meningitis or spondylitis: an analysis of eight cases. Rheumatology (Oxford). 2004;43(12):1508–12. https://doi.org/10.1093/rheumatology/keh370.
Haikal A, Everist BM, Jetanalin P, Maz M. Cervical CT-dependent diagnosis of crowned dens syndrome in calcium pyrophosphate dihydrate crystal deposition disease. Am J Med. 2020;133(2):e32–7. https://doi.org/10.1016/j.amjmed.2019.06.050.
• Ziegeler K, Diekhoff T, Hermann S, Hamm B, Hermann KGA. Low-dose computed tomography as diagnostic tool in calcium pyrophosphate deposition disease arthropathy: focus on ligamentous calcifications of the wrist. Clin Exp Rheumatol. 2019;37(5):826–33. This was a study that showed that low-dose CT can be effective in diagnosing CPPD arthropathy, thereby decreasing the amount of radiation exposure to patients.
•• Døssing A, Müller FC, Becce F, Stamp L, Bliddal H, Boesen M. Dual-energy computed tomography for detection and characterization of monosodium urate, calcium pyrophosphate, and hydroxyapatite: a phantom study on diagnostic performance. Invest Radiol. 2021. https://doi.org/10.1097/rli.0000000000000756. This was an important recent study that showed how dual-energy CT under standard imaging settings, can be used to differentiate between different forms of crystal arthropathy, particularly monosodium urate deposition (gout), calcium pyrophosphate deposition, and hydroxyapatite deposition, thereby making it a key noninvasive imaging modality to help differentiate between common crystal arthropathies.
Tedeschi SK, Solomon DH, Yoshida K, Vanni K, Suh DH, Smith SE. A prospective study of dual-energy CT scanning, US and X-ray in acute calcium pyrophosphate crystal arthritis. Rheumatology (Oxford). 2020;59(4):900–3. https://doi.org/10.1093/rheumatology/kez431.
•• Budzik JF, Marzin C, Legrand J, Norberciak L, Becce F, Pascart T. Can dual-energy computed tomography be used to identify early calcium crystal deposition in the knees of patients with calcium pyrophosphate deposition? Arthritis Rheumatol. 2021;73(4):687–92. https://doi.org/10.1002/art.41569. This was another important recent study on dual-energy CT that reinforced its ability in detecting CPP deposition in the knees before it is picked up on plain radiographs, thereby allowing for earlier non-invasive diagnosis.
Rougé-Labriet H, Berujon S, Mathieu H, Bohic S, Fayard B, Ravey JN, et al. X-ray Phase Contrast osteo-articular imaging: a pilot study on cadaveric human hands. Sci Rep. 2020;10(1):1911. https://doi.org/10.1038/s41598-020-58168-3.
Finkenstaedt T, Biswas R, Abeydeera NA, Siriwanarangsun P, Healey R, Statum S, et al. Ultrashort time to echo magnetic resonance evaluation of calcium pyrophosphate crystal deposition in human menisci. Invest Radiol. 2019;54(6):349–55. https://doi.org/10.1097/rli.0000000000000547.
Suan JC, Chhem RK, Gati JS, Norley CJ, Holdsworth DW. 4 T MRI of chondrocalcinosis in combination with three-dimensional CT, radiography, and arthroscopy: a report of three cases. Skeletal Radiol. 2005;34(11):714–21. https://doi.org/10.1007/s00256-005-0930-y.
Moshrif A, Laredo JD, Bassiouni H, Abdelkareem M, Richette P, Rigon MR, et al. Spinal involvement with calcium pyrophosphate deposition disease in an academic rheumatology center: a series of 37 patients. Semin Arthritis Rheum. 2019;48(6):1113–26. https://doi.org/10.1016/j.semarthrit.2018.10.009.
Li B, Singer NG, Yeni YN, Haggins DG, Barnboym E, Oravec D, et al. A Point-of-care Raman spectroscopy-based device for the diagnosis of gout and pseudogout: comparison with the clinical standard microscopy. Arthritis Rheumatol. 2016;68(7):1751–7. https://doi.org/10.1002/art.39638.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that this article complies with ethics guidelines.
Conflict of Interest
JS reports no conflicts of interest. MHP is supported in part by grant 2UL1 TR001445-06A1 from the National Center for the Advancement of Translational Science of the National Institutes of Health, has received investigator-initiated grants from Horizon Therapeutics and Hikma Pharmaceuticals, and has served as a consultant for Horizon Therapeutics. MT has received investigator-initiated grants from Horizon Therapeutics and has served as a consultant for Horizon Therapeutics.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Imaging
Rights and permissions
About this article
Cite this article
Sullivan, J., Pillinger, M.H. & Toprover, M. Chondrocalcinosis: Advances in Diagnostic Imaging. Curr Rheumatol Rep 23, 77 (2021). https://doi.org/10.1007/s11926-021-01044-4
Accepted:
Published:
DOI: https://doi.org/10.1007/s11926-021-01044-4