Abstract
Purpose of Review
To date, a vast amount of information regarding ubiquitination (Ub) and ubiquitylation-like (Ubl) modification–related mechanisms has been reported in the context of skeletal cell homeostasis and diseases. In this review, we mainly focus on recent findings regarding the contribution of enzymatic machinery that directly adds or removes Ub and Ubl modifications from protein targets in chondrocyte homeostasis and osteoarthritis (OA) development.
Recent Findings
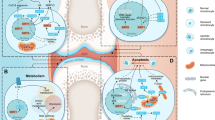
Mechanisms that promote homeostasis of articular chondrocytes are crucial for maintaining the integrity of articular joints to prevent osteoarthritis development. Articular chondrocytes are postmitotic cells that continuously produce and remodel cartilage matrix. In addition, the long lifespan of chondrocytes makes them susceptible to accumulating cellular damage. Ub and the evolutionarily conserved Ubl modifications, such as SUMOylation, ATGylation, and UFMylation, play important roles in promoting chondrocyte homeostasis, including regulating cell signaling and protein stability, resolving cellular stresses and inflammation, and maintaining differentiation and survival of chondrocytes.
Summary
Uncovering new components/functions of Ub/Ubl modification machinery may provide novel drug targets to treat OA.

Similar content being viewed by others
References
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–707. https://doi.org/10.1002/art.34453.
He B, Wu JP, Kirk TB, Carrino JA, Xiang C, Xu J. High-resolution measurements of the multilayer ultra-structure of articular cartilage and their translational potential. Arthritis Res Ther. 2014;16:205. https://doi.org/10.1186/ar4506.
Zahan O-M, Serban O, Gherman C, Fodor D. The evaluation of oxidative stress in osteoarthritis. Med Pharm Rep. 2020;93:12–22. https://doi.org/10.15386/mpr-1422.
Tan L, Register TC, Yammani RR. Age-related decline in expression of molecular chaperones induces endoplasmic reticulum stress and chondrocyte apoptosis in articular cartilage. Aging Dis. 2020;11:1091–102. https://doi.org/10.14336/AD.2019.1130.
Greene MA, Loeser RF. Aging-related inflammation in osteoarthritis. Osteoarthr Cartil. 2015;23:1966–71. https://doi.org/10.1016/j.joca.2015.01.008.
Rellmann Y, Eidhof E, Dreier R. Review: ER stress-induced cell death in osteoarthritic cartilage. Cell Signal. 2021;78:109880. https://doi.org/10.1016/j.cellsig.2020.109880.
Griffin TM, Guilak F. The role of mechanical loading in the onset and progression of osteoarthritis. Exerc Sport Sci Rev. 2005;33:195–200. https://doi.org/10.1097/00003677-200510000-00008.
Hershko A, Ciechanover A, Varshavsky A. Basic Medical Research Award. The ubiquitin system. Nat Med. 2000;6:1073–81. https://doi.org/10.1038/80384.
Buetow L, Huang DT. Structural insights into the catalysis and regulation of E3 ubiquitin ligases. Nat Rev Mol Cell Biol. 2016;17:626–42. https://doi.org/10.1038/nrm.2016.91.
Popovic D, Vucic D, Dikic I. Ubiquitination in disease pathogenesis and treatment. Nat Med. 2014;20:1242–53. https://doi.org/10.1038/nm.3739.
George AJ, Hoffiz YC, Charles AJ, Zhu Y, Mabb AM. A comprehensive atlas of E3 ubiquitin ligase mutations in neurological disorders. Front Genet. 2018;9:29. https://doi.org/10.3389/fgene.2018.00029.
Fan L, Bi T, Wang L, Xiao W. DNA-damage tolerance through PCNA ubiquitination and sumoylation. Biochem J. 2020;477:2655–77. https://doi.org/10.1042/BCJ20190579.
Huangfu W-C, Fuchs SY. Ubiquitination-dependent regulation of signaling receptors in cancer. Genes Cancer. 2010;1:725–34. https://doi.org/10.1177/1947601910382901.
Imamura T, Oshima Y, Hikita A. Regulation of TGF-β family signalling by ubiquitination and deubiquitination. J Biochem (Tokyo). 2013;154:481–9. https://doi.org/10.1093/jb/mvt097.
Han M-S, Jung Y-K, Kim G-W, Han S. Transglutaminase-2 regulates Wnt and FoxO3a signaling to determine the severity of osteoarthritis. Sci Rep. 2020;10:13228. https://doi.org/10.1038/s41598-020-70115-w.
Ba C, Ni X, Yu J, Zou G, Zhu H. Ubiquitin conjugating enzyme E2 M promotes apoptosis in osteoarthritis chondrocytes via Wnt/β-catenin signaling. Biochem Biophys Res Commun. 2020;529:970–6. https://doi.org/10.1016/j.bbrc.2020.06.095.
Wu C-J, Conze DB, Li T, Srinivasula SM, Ashwell JD. Nat Cell Biol. 2006;8:398–406. https://doi.org/10.1038/ncb1384.
Serrano RL, Chen L-Y, Lotz MK, Liu-Bryan R, Terkeltaub R. Impaired proteasomal function in human osteoarthritic chondrocytes can contribute to decreased levels of SOX9 and aggrecan. Arthritis Rheumatol Hoboken NJ. 2018;70:1030–41. https://doi.org/10.1002/art.40456.
Liang C, Liang G, Zheng X, Huang Y, Huang S, Yin D. RSP5 positively regulates the osteogenic differentiation of mesenchymal stem cells by activating the K63-linked ubiquitination of Akt. Stem Cells Int. 2020;2020:7073805. https://doi.org/10.1155/2020/7073805.
Guo L, Wang R, Zhang K, Yuan J, Wang J, Wang X, et al. A PINCH-1-Smurf1 signaling axis mediates mechano-regulation of BMPR2 and stem cell differentiation. J Cell Biol. 2019;218:3773–94. https://doi.org/10.1083/jcb.201902022.
Zhang W, Hou W, Chen M, Chen E, Xue D, Ye C, et al. Upregulation of Parkin accelerates osteoblastic differentiation of bone marrow-derived mesenchymal stem cells and bone regeneration by enhancing autophagy and β-catenin signaling. Front Cell Dev Biol. 2020;8:576104. https://doi.org/10.3389/fcell.2020.576104.
Xu L, Wu Z, He Y, Chen Z, Xu K, Yu W, et al. MFN2 contributes to metabolic disorders and inflammation in the aging of rat chondrocytes and osteoarthritis. Osteoarthr Cartil. 2020;28:1079–91. https://doi.org/10.1016/j.joca.2019.11.011.
Wang G, Chen S, Xie Z, Shen S, Xu W, Chen W, et al. TGFβ attenuates cartilage extracellular matrix degradation via enhancing FBXO6-mediated MMP14 ubiquitination. Ann Rheum Dis. 2020;79:1111–20. https://doi.org/10.1136/annrheumdis-2019-216911.
Mokuda S, Nakamichi R, Matsuzaki T, Ito Y, Sato T, Miyata K, et al. Wwp2 maintains cartilage homeostasis through regulation of Adamts5. Nat Commun. 2019;10:2429. https://doi.org/10.1038/s41467-019-10177-1.
Li K, Yang P, Zhang Y, Zhang Y, Cao H, Liu P, et al. DEPTOR prevents osteoarthritis development via interplay with TRC8 to reduce endoplasmic reticulum stress in chondrocytes. J Bone Miner Res Off J Am Soc Bone Miner Res. 2020. https://doi.org/10.1002/jbmr.4176.
Yang L, Wang Z, Zou C, Mi Y, Tang H, Wu X. Ubiquitin-specific protease 49 attenuates IL-1β-induced rat primary chondrocyte apoptosis by facilitating Axin deubiquitination and subsequent Wnt/β-catenin signaling cascade inhibition. Mol Cell Biochem. 2020;474:263–75. https://doi.org/10.1007/s11010-020-03850-3.
Zhou Q, Xiao Z, Zhou R, Zhou Y, Fu P, Li X, et al. Ubiquitin-specific protease 3 targets TRAF6 for deubiquitination and suppresses IL-1β induced chondrocyte apoptosis. Biochem Biophys Res Commun. 2019;514:482–9. https://doi.org/10.1016/j.bbrc.2019.04.163.
Wilkinson KA, Henley JM. Mechanisms, regulation and consequences of protein SUMOylation. Biochem J. 2010;428:133–45. https://doi.org/10.1042/BJ20100158.
Liang Y-C, Lee C-C, Yao Y-L, Lai C-C, Schmitz ML, Yang W-M. SUMO5, a novel poly-SUMO isoform, regulates PML nuclear bodies. Sci Rep. 2016;6:26509. https://doi.org/10.1038/srep26509.
Sarge KD, Park-Sarge O-K. Sumoylation and human disease pathogenesis. Trends Biochem Sci. 2009;34:200–5. https://doi.org/10.1016/j.tibs.2009.01.004.
Hay RT. SUMO: a history of modification. Mol Cell. 2005;18:1–12. https://doi.org/10.1016/j.molcel.2005.03.012.
Celen AB, Sahin U. Sumoylation on its 25th anniversary: mechanisms, pathology, and emerging concepts. FEBS J. 2020;287:3110–40. https://doi.org/10.1111/febs.15319.
Akiyama H, Chaboissier M-C, Martin JF, Schedl A, de Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–28. https://doi.org/10.1101/gad.1017802.
Lefebvre V, Behringer RR, de Crombrugghe B. L-Sox5, Sox6 and Sox9 control essential steps of the chondrocyte differentiation pathway. Osteoarthr Cartil. 2001;9(Suppl A):S69–75. https://doi.org/10.1053/joca.2001.0447.
Liu C-F, Lefebvre V. The transcription factors SOX9 and SOX5/SOX6 cooperate genome-wide through super-enhancers to drive chondrogenesis. Nucleic Acids Res. 2015;43:8183–203. https://doi.org/10.1093/nar/gkv688.
Hattori T, Eberspaecher H, Lu J, Zhang R, Nishida T, Kahyo T, et al. Interactions between PIAS proteins and SOX9 result in an increase in the cellular concentrations of SOX9. J Biol Chem. 2006;281:14417–28. https://doi.org/10.1074/jbc.M511330200.
Pei H, Chen L, Liao Q-M, Wang K-J, Chen S-G, Liu Z-J, et al. SUMO-specific protease 2 (SENP2) functions as a tumor suppressor in osteosarcoma via SOX9 degradation. Exp Ther Med. 2018;16:5359–65. https://doi.org/10.3892/etm.2018.6838.
Grossmann KS, Rosário M, Birchmeier C, Birchmeier W. The tyrosine phosphatase Shp2 in development and cancer. Adv Cancer Res. 2010;106:53–89. https://doi.org/10.1016/S0065-230X(10)06002-1.
Neel BG, Gu H, Pao L. The ‘Shp’ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–93. https://doi.org/10.1016/S0968-0004(03)00091-4.
Zuo C, Wang L, Kamalesh RM, Bowen ME, Moore DC, Dooner MS, et al. SHP2 regulates skeletal cell fate by modifying SOX9 expression and transcriptional activity. Bone Res. 2018;6:12. https://doi.org/10.1038/s41413-018-0013-z.
Oh HJ, Kido T, Lau Y-FC. PIAS1 interacts with and represses SOX9 transactivation activity. Mol Reprod Dev. 2007;74:1446–55. https://doi.org/10.1002/mrd.20737.
Fernández-Lloris R, Osses N, Jaffray E, Shen LN, Vaughan OA, Girwood D, et al. Repression of SOX6 transcriptional activity by SUMO modification. FEBS Lett. 2006;580:1215–21. https://doi.org/10.1016/j.febslet.2006.01.031.
Im H-J, Sharrocks AD, Lin X, Yan D, Kim J, van Wijnen AJ, et al. Basic fibroblast growth factor induces matrix metalloproteinase-13 via ERK MAP kinase-altered phosphorylation and sumoylation of Elk-1 in human adult articular chondrocytes. Open Access Rheumatol Res Rev. 2009;1:151–61. https://doi.org/10.2147/oarrr.s7527.
Choi H-J, Kwon S, Kim D-W. A post-translational modification cascade employing HDAC9-PIASy-RNF4 axis regulates chondrocyte hypertrophy by modulating Nkx3.2 protein stability. Cell Signal. 2016;28:1336–48. https://doi.org/10.1016/j.cellsig.2016.06.006.
arcOGEN Consortium, arcOGEN Collaborators, Zeggini E, Panoutsopoulou K, Southam L, Rayner NW, et al. Identification of new susceptibility loci for osteoarthritis (arcOGEN): a genome-wide association study. Lancet Lond Engl. 2012;380:815–23. https://doi.org/10.1016/S0140-6736(12)60681-3.
Johnson K, Reynard LN, Loughlin J. Functional characterisation of the osteoarthritis susceptibility locus at chromosome 6q14.1 marked by the polymorphism rs9350591. BMC Med Genet. 2015;16:81. https://doi.org/10.1186/s12881-015-0215-9.
Li J, Lu D, Dou H, Liu H, Weaver K, Wang W, et al. Desumoylase SENP6 maintains osteochondroprogenitor homeostasis by suppressing the p53 pathway. Nat Commun. 2018;9:143. https://doi.org/10.1038/s41467-017-02413-3.
Zhu B, Cui G, Zhang Q, Cheng X, Tang S. Desumoylation of aggrecan and collagen II facilitates degradation via aggrecanases in IL-1β-mediated osteoarthritis. J Pain Res. 2019;12:2145–53. https://doi.org/10.2147/JPR.S194306.
Chen R-H, Chen Y-H, Huang T-Y. Ubiquitin-mediated regulation of autophagy. J Biomed Sci. 2019;26:80. https://doi.org/10.1186/s12929-019-0569-y.
Nakatogawa H. Two ubiquitin-like conjugation systems that mediate membrane formation during autophagy. Essays Biochem. 2013;55:39–50. https://doi.org/10.1042/bse0550039.
Caramés B, Taniguchi N, Otsuki S, Blanco FJ, Lotz M. Autophagy is a protective mechanism in normal cartilage, and its aging-related loss is linked with cell death and osteoarthritis. Arthritis Rheum. 2010;62:791–801. https://doi.org/10.1002/art.27305.
Zhang Y, Vasheghani F, Li Y-H, Blati M, Simeone K, Fahmi H, et al. Cartilage-specific deletion of mTOR upregulates autophagy and protects mice from osteoarthritis. Ann Rheum Dis. 2015;74:1432–40. https://doi.org/10.1136/annrheumdis-2013-204599.
Shen C, Cai G-Q, Peng J-P, Chen X-D. Autophagy protects chondrocytes from glucocorticoids-induced apoptosis via ROS/Akt/FOXO3 signaling. Osteoarthr Cartil. 2015;23:2279–87. https://doi.org/10.1016/j.joca.2015.06.020.
Bouderlique T, Vuppalapati KK, Newton PT, Li L, Barenius B, Chagin AS. Targeted deletion of Atg5 in chondrocytes promotes age-related osteoarthritis. Ann Rheum Dis. 2016;75:627–31. https://doi.org/10.1136/annrheumdis-2015-207742.
Vuppalapati KK, Bouderlique T, Newton PT, Kaminskyy VO, Wehtje H, Ohlsson C, et al. Targeted deletion of autophagy genes Atg5 or Atg7 in the chondrocytes promotes caspase-dependent cell death and leads to mild growth retardation. J Bone Miner Res Off J Am Soc Bone Miner Res. 2015;30:2249–61. https://doi.org/10.1002/jbmr.2575.
Liao F-X, Huang F, Ma W-G, Qin K-P, Xu P-F, Wu Y-F, et al. The new role of Sirtuin1 in human osteoarthritis chondrocytes by regulating autophagy. Cartilage. 2019:1947603519847736. https://doi.org/10.1177/1947603519847736.
Sacitharan PK, Bou-Gharios G, Edwards JR. SIRT1 directly activates autophagy in human chondrocytes. Cell Death Dis. 2020;6:41. https://doi.org/10.1038/s41420-020-0277-0.
Shah K, Cai H, Lane JCE, Collins GS, Arden NK, Furniss D, et al. Prognostic factors for finger interphalangeal joint osteoarthritis: a systematic review. Rheumatol Oxf Engl. 2020. https://doi.org/10.1093/rheumatology/keaa735.
Wu C, Zheng J, Yao X, Shan H, Li Y, Xu P, et al. Defective autophagy in chondrocytes with Kashin-Beck disease but higher than osteoarthritis. Osteoarthr Cartil. 2014;22:1936–46. https://doi.org/10.1016/j.joca.2014.08.010.
Wu C, Wen Y, Guo X, Yang T, Shen H, Chen X, et al. Genetic association, mRNA and protein expression analysis identify ATG4C as a susceptibility gene for Kashin-Beck disease. Osteoarthr Cartil. 2017;25:281–6. https://doi.org/10.1016/j.joca.2016.09.019.
He W, Cheng Y. Inhibition of miR-20 promotes proliferation and autophagy in articular chondrocytes by PI3K/AKT/mTOR signaling pathway. Biomed Pharmacother Biomedecine Pharmacother. 2018;97:607–15. https://doi.org/10.1016/j.biopha.2017.10.152.
D’Adamo S, Alvarez-Garcia O, Muramatsu Y, Flamigni F, Lotz MK. MicroRNA-155 suppresses autophagy in chondrocytes by modulating expression of autophagy proteins. Osteoarthr Cartil. 2016;24:1082–91. https://doi.org/10.1016/j.joca.2016.01.005.
Lian W-S, Ko J-Y, Wu R-W, Sun Y-C, Chen Y-S, Wu S-L, et al. MicroRNA-128a represses chondrocyte autophagy and exacerbates knee osteoarthritis by disrupting Atg12. Cell Death Dis. 2018;9:919. https://doi.org/10.1038/s41419-018-0994-y.
Yu Y, Zhao J. Modulated autophagy by microRNAs in osteoarthritis chondrocytes. Biomed Res Int. 2019;2019:1484152. https://doi.org/10.1155/2019/1484152.
Xu R, Wei Y, Yin X, Shi B, Li J. Author Correction: miR-20a suppresses chondrogenic differentiation of ATDC5 cells by regulating Atg7. Sci Rep. 2020;10:1179. https://doi.org/10.1038/s41598-020-58140-1.
Watson CM, Crinnion LA, Gleghorn L, Newman WG, Ramesar R, Beighton P, et al. Identification of a mutation in the ubiquitin-fold modifier 1-specific peptidase 2 gene, UFSP2, in an extended South African family with Beukes hip dysplasia. South Afr Med J Suid-Afr Tydskr Vir Geneeskd. 2015;105:558–63. https://doi.org/10.7196/SAMJnew.7917.
Di Rocco M, Rusmini M, Caroli F, Madeo A, Bertamino M, Marre-Brunenghi G, et al. Novel spondyloepimetaphyseal dysplasia due to UFSP2 gene mutation. Clin Genet. 2018;93:671–4. https://doi.org/10.1111/cge.13134.
Zhang G, Tang S, Wang H, Pan H, Zhang W, Huang Y, et al. UFSP2-related spondyloepimetaphyseal dysplasia: a confirmatory report. Eur J Med Genet. 2020;63:104021. https://doi.org/10.1016/j.ejmg.2020.104021.
Egunsola AT, Bae Y, Jiang M-M, Liu DS, Chen-Evenson Y, Bertin T, et al. Loss of DDRGK1 modulates SOX9 ubiquitination in spondyloepimetaphyseal dysplasia. J Clin Invest. 2017;127:1475–84. https://doi.org/10.1172/JCI90193.
Yang G, Wang Y, Chen Y, Huang R. UFL1 attenuates IL-1β-induced inflammatory response in human osteoarthritis chondrocytes. Int Immunopharmacol. 2020;81:106278. https://doi.org/10.1016/j.intimp.2020.106278.
Villarroya-Beltri C, Guerra S, Sánchez-Madrid F. ISGylation - a key to lock the cell gates for preventing the spread of threats. J Cell Sci. 2017;130:2961–9. https://doi.org/10.1242/jcs.205468.
Adapala NS, Swarnkar G, Arra M, Shen J, Mbalaviele G, Ke K, et al. Inflammatory osteolysis is regulated by site-specific ISGylation of the scaffold protein NEMO. ELife. 2020;9. https://doi.org/10.7554/eLife.56095.
Liu K, Chen K, Zhang Q, Zhang L, Yan Y, Guo C, et al. TRAF6 neddylation drives inflammatory arthritis by increasing NF-κB activation. Lab Investig J Tech Methods Pathol. 2019;99:528–38. https://doi.org/10.1038/s41374-018-0175-8.
Chan C-H, Morrow JK, Li C-F, Gao Y, Jin G, Moten A, et al. Pharmacological inactivation of Skp2 SCF ubiquitin ligase restricts cancer stem cell traits and cancer progression. Cell. 2013;154:556–68. https://doi.org/10.1016/j.cell.2013.06.048.
Funding
The paper is supported by funding NIH/NIA R01AG061086 and the Arthritis National Research Foundation (ANRF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Osteoarthritis
Rights and permissions
About this article
Cite this article
Liu, Y., Molchanov, V. & Yang, T. Enzymatic Machinery of Ubiquitin and Ubiquitin-Like Modification Systems in Chondrocyte Homeostasis and Osteoarthritis. Curr Rheumatol Rep 23, 62 (2021). https://doi.org/10.1007/s11926-021-01022-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s11926-021-01022-w